Sabin Vaccine Institute’s Investigational Marburg Vaccine Delivered to Ethiopia for Outbreak Respons
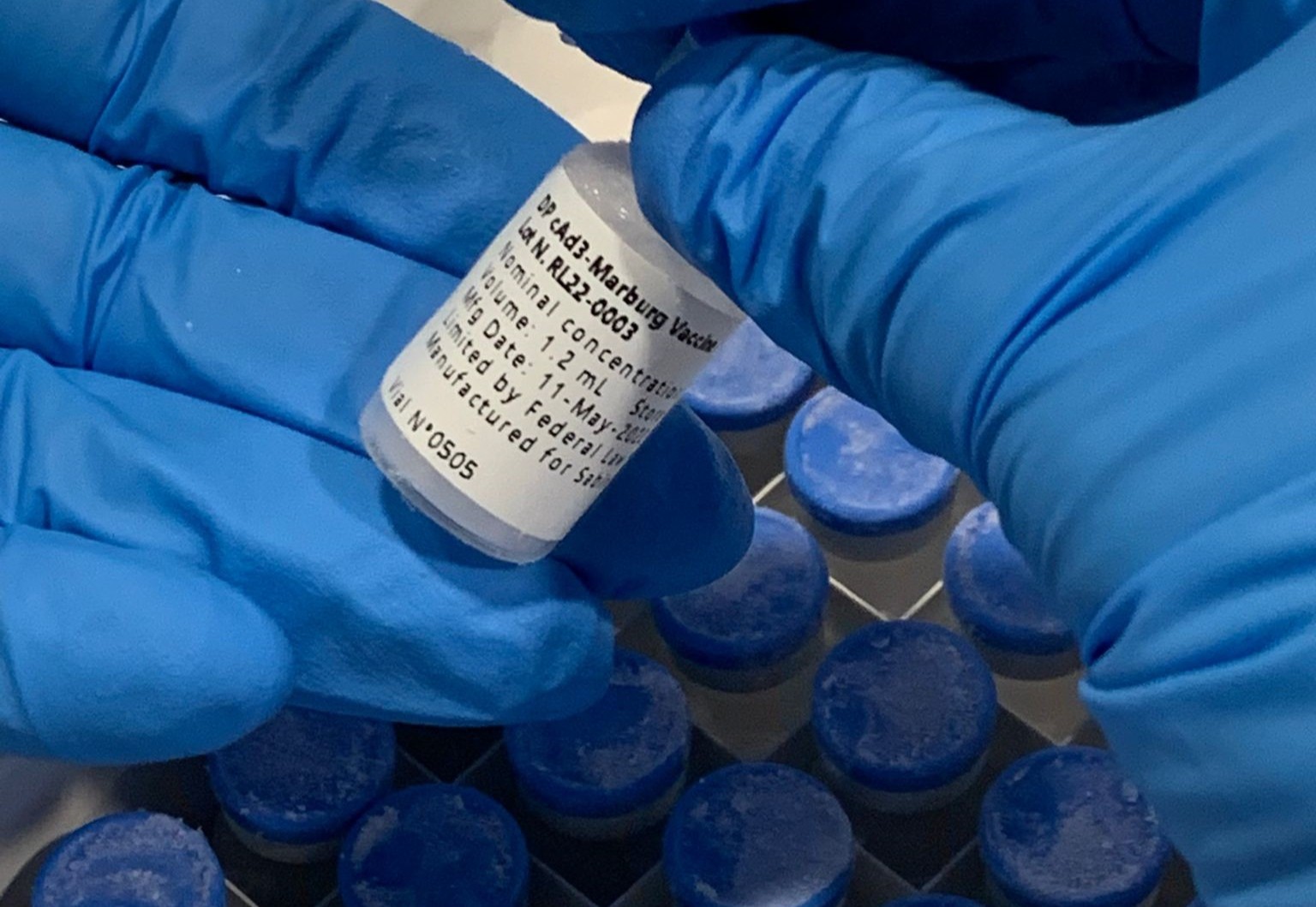
WASHINGTON, Dec. 04, 2025 (GLOBE NEWSWIRE) -- The Sabin Vaccine Institute (Sabin) has sent more than 640 doses of its investigational cAd3-Marburg Vaccine to Ethiopia to support the country’s response to its first-ever outbreak of Marburg virus disease. Marburg is a highly contagious hemorrhagic fever disease and can have a high case fatality rate of up to 88%. There are currently no licensed vaccines or treatments for Marburg.
Soon after Marburg was confirmed as the virus causing a hemorrhagic fever outbreak in Ethiopia’s southern region, the Ethiopia Ministry of Health engaged Sabin and the U.S. government to request access to Sabin’s investigational cAd3-Marburg Vaccine. The U.S. government approved this request. The Biomedical Advanced Research and Development Authority (BARDA), part of U.S. Department of Health and Human Services’ Administration for Strategic Preparedness and Response (ASPR), funds the development and manufacture of Sabin’s investigational vaccine candidate.
Sabin and the Ethiopia Ministry of Health have entered into a clinical trial agreement under which Sabin is providing BARDA-funded investigational cAd3-Marburg Vaccine doses for a two-cohort Phase 2, rapid response, open-label, randomized trial to assess safety, efficacy, and immunogenicity. Under this approved protocol, Cohort A is limited to high-risk health care and front-line workers or individuals who have had direct contact with infected persons within 21 days, the incubation period for Marburg virus disease. All other health care and front-line workers and contacts will be randomized in Cohort B, some receiving vaccine on Day 1 and others on Day 22 of the trial.
“The Ethiopian Ministry of Health reached out to Sabin early in the outbreak, and we have been working in partnership to support Minister of Health Dr. Mekdes Daba and her team,” says Sabin Chief Executive Officer Amy Finan. “We’ve built on our previous outbreak response experience to quickly assist our Ethiopian colleagues as requested.”
In addition to coordinating directly with the Minister of Health and the Armauer Hansen Research Institute (AHRI), Sabin is also working closely with manufacturing partner ReiThera, clinical research organization IQVIA and the Coalition for Epidemic Preparedness Innovations (CEPI) – the same organizations that came together to support Rwanda’s response to their 2024 outbreak. With authorization from ASPR, Sabin provided cAd3-Marburg Vaccine to the Rwanda Biomedical Center for use in a Phase 2 clinical trial.
Ethiopia’s Ministry of Health has confirmed 13 Marburg infections, including eight deaths, in their most recent update. Another three deaths are suspected but not confirmed. Officials continue to express concern about the outbreak’s proximity to fragile health settings in nearby Kenya and South Sudan.
In addition to Sabin’s single-dose cAd3-Marburg Vaccine being used in a Phase 2 outbreak clinical trial in Rwanda, the vaccine is also in Phase 2 clinical trials in the U.S., Uganda and Kenya. More than 2000 trial participants have received the vaccine and no significant safety concerns have been reported. Results from Phase 1 clinical trials and nonclinical studies indicate that the vaccine is safe and elicits rapid, robust immune responses.
The Sabin cAd3-Marburg Vaccine doses have been supported in whole or in part with federal funds from the U.S. Department of Health and Human Services; Administration for Strategic Preparedness and Response; Biomedical Advanced Research and Development Authority (BARDA), under contract numbers 75A50119C00055 and 75A50123C00010.
Once rare, Marburg virus disease outbreaks have surged in Africa in recent years, with incidents reported in 2023 in Tanzania and Equatorial Guinea, in 2024 in Rwanda and in 2025 in Tanzania. Marburg is transmitted from fruit bats to humans, spreading from person to person through contact with infected bodily fluids.
About the Sabin Vaccine Institute
The Sabin Vaccine Institute is a leading advocate for expanding vaccine access and uptake globally, advancing vaccine research and development, and amplifying vaccine knowledge and innovation. Unlocking the potential of vaccines through partnership, Sabin has built a robust ecosystem of funders, innovators, implementers, practitioners, policy makers and public stakeholders to advance its vision of a future free from preventable diseases. As a non-profit with three decades of experience, Sabin is committed to finding solutions that last and extending the full benefits of vaccines to all people, regardless of who they are or where they live. At Sabin, we believe in the power of vaccines to change the world. For more information, visit www.sabin.org and follow us on X, @SabinVaccine.
About the cAd3 Platform
In August 2019, Sabin announced exclusive agreements with GSK for Sabin to advance the development of the prophylactic candidate vaccines against the deadly Zaire ebolavirus, Sudan virus, and Marburg virus. The three candidate vaccines were initially developed collaboratively by the U.S. National Institutes of Health and Okairos, which was acquired by GSK in 2013. The candidate vaccines, based on GSK’s proprietary cAd3 (Chimpanzee Adenovirus Type 3) platform, were further developed by GSK, including the Phase 2 development for the Zaire ebolavirus vaccine. Under the agreements between GSK and Sabin, Sabin exclusively licensed the technology for all three candidate vaccines and acquired certain patent rights specific to these vaccines. Sabin is developing the cAd3-Marburg Vaccine and cAd3-Sudan Vaccine, both are in Phase 2 clinical trials in the US and Africa.
Media Contact:
Monika Guttman
Media Relations Specialist
Sabin Vaccine Institute
+1 (202) 662-1841
press@sabin.org
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/c29e0887-de65-45d0-9d2f-4365a8bc78ea
- 学术盛宴圆满落幕!第五届闽江眼底病论坛:以创新之力续写疑难眼病探索新篇
- 杭州威雅学校:准备好了吗?!2024杭州威雅音乐节蓄势待发
- 苏打绿阿龚首次个人巡回音乐会掀口碑,巡回限定《小宇宙》钢琴串烧引全场回忆潮
- 探索手游的无限可能:优质手游戏平台与游戏代理的重要性
- AAVantgarde to Present Updated Clinical Data from LUCE-1 Study at EURetina 2025
- 新闻动态|碳收侠APP与赛宝认证携手绘就新蓝图!
- 丰尚农牧参加2024年中非合作论坛北京峰会
- Esri发布其新版畅销GIS地图设计指南
- 越秀北京 梧桐星宸|年轻有“位” super85m²,安家就爱这个范儿
- 把关爱和温暖送到退役军人的心坎上!福州“百城千企暖兵心”困难帮扶行动启动
- AB InBev Reports Second Quarter 2024 Results
- 用第一做第一,晶科能源的发展逻辑
- FPT Announced a Partner Ecosystem with Global Tech Giants, Promoting AI Factory Development and Oper
- 2024年北京十大杰出律师事务所排名概览
- 富士康集团成员鸿准精密斥资3000万美元注资博歌科技,共拓医疗机器人护老市场
- 桂林嘉城商贸:门窗工程里的服务与细节 “协奏曲”
- 优艾智合机器人强势入围2025 IERA大奖
- 哪类人群更适合做飞秒激光白内障手术?爱尔英智眼科医院丁雪为您讲解
- 九识智能助力甘肃邮政快递行业职业技能大赛,共绘智慧物流新蓝图
- 从脑机芯片到气候变化:2024年迪拜未来论坛预测人类未来
- 国际通行 品质保障 ——山东安华生物医药股份有限公司零缺陷通过美国FDA审查!
- 横河电机与丰田签署载人加压月球车控制平台研发协议
- 重磅!睿笛生物首创纳秒级陡脉冲恶性实体瘤消融系统获批上市
- 【新闻动态】如何加快“双碳”人才队伍建设暨碳收侠App“双碳”测评办公室成立
- “致敬百年、续写新篇”王天禾中国画艺术研讨展在京隆重开幕
- Chainstack Secures Strategic Investment to Accelerate Web3 Infrastructure Development
- 农发行衡阳市分行:深入学习,精准施策,全面贯彻政策性金融支持乡村振兴政策
- Bitget Ranks #2 Globally in Monthly CEX Inflows, Attracting $1.78 Billion, DefiLlama Confirms
- 重磅官宣!实力演员董璇携手维萃美,开启科学养护新篇章
- 双十一期间,艾芬达如何以智能卫浴产品引领健康生活潮流?
推荐
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
 透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
-
 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
-
 周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
-
 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
-
 新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯

