Zymeworks Appoints Scott Platshon as Acting Chief Investment Officer

VANCOUVER, British Columbia, Nov. 18, 2025 (GLOBE NEWSWIRE) -- Zymeworks Inc. (Nasdaq: ZYME), a biotechnology company managing a portfolio of licensed healthcare assets, while developing a diverse pipeline of novel, multifunctional biotherapeutics today announced the appointment of Scott Platshon as Acting Chief Investment Officer.
Mr. Platshon will report directly to Zymeworks’ Chair and Chief Executive Officer, Kenneth Galbraith, and work closely with him to manage expected future cash flows from Ziihera® (zanidatamab-hrii) and other healthcare assets and licensed product candidates, such as pasritamig, which is being advanced to Phase 3 registration studies by Johnson & Johnson Innovative Medicine. He will also manage the operational execution of Zymeworks’ healthcare asset aggregation strategy.
Concurrent with this appointment, Mr. Platshon has stepped down from the Company’s Board of Directors. As this is a part-time role, he will also continue to serve as a Partner at EcoR1 Capital, LLC, a biotech-focused investment fund.
“Following the positive topline data from the Phase 3 HERIZON-GEA-01 trial evaluating zanidatamab in combination with chemotherapy with or without the PD-1 inhibitor Tevimbra® (tislelizumab), we are strategically positioned to drive long-term returns by building a diversified portfolio of revenue-generating assets,” said Mr. Galbraith. “Scott has played a key role in managing EcoR1’s investment in Zymeworks, including joining our Board in February 2024. He has been working closely with me and the Board to complement our active R&D operations with a novel strategic initiative of actively managing and aggregating revenue-generating assets to generate attractive long-term shareholder returns from an integrated business approach. Scott’s deep investment expertise, in-depth knowledge of the Company and our strategic objectives, and global network make him uniquely suited to help accelerate these efforts and protect and manage value arising from our royalty portfolio.”
Mr. Platshon has worked at EcoR1 Capital since 2015, and served as a Partner since 2020. Prior to joining the firm, he was an analyst at Aquilo Partners, a San Francisco-based boutique life sciences investment bank. Mr. Platshon also sits on the board of directors of Kumquat Biosciences and Ajax Therapeutics. Mr. Platshon holds a B.S. in Bioengineering from Stanford University.
“I am eager to continue working closely with Zymeworks, now in this new role, at such a pivotal moment, and share in the Company’s unified strategic vision to pursue innovation and growth,” said Scott Platshon. “The Company has built a strong financial foundation with Ziihera, its broader platform collaborations and wholly-owned R&D portfolio, and I see tremendous opportunity to leverage these cash flows to build a disciplined, high-return portfolio that creates sustainable value creation over the long-term. I look forward to enhancing my involvement within the Zymeworks team and forging new capital streams to deliver meaningful returns for shareholders.”
About Zymeworks Inc.
Zymeworks is a global biotechnology company managing a portfolio of licensed healthcare assets and developing a diverse pipeline of novel, multifunctional biotherapeutics to improve the standard of care for difficult-to-treat diseases, including cancer, inflammation, and autoimmune disease. The Company’s asset and royalty aggregation strategy focuses on optimizing positive future cash flows from an emerging portfolio of licensed products such as Ziihera® (zanidatamab-hrii), pasritamig, and other licensed products and product candidates, such as pasritamig. In addition, Zymeworks is also building a portfolio of healthcare assets that can generate strong cash flows, while supporting the early-stage development of innovative medicines. Zymeworks engineered and developed Ziihera® (zanidatamab-hrii), a HER2-targeted bispecific antibody using the Company’s proprietary Azymetric™ technology and has entered into separate agreements with BeOne Medicines Ltd. (formerly BeiGene, Ltd.) and Jazz Pharmaceuticals Ireland Limited granting each exclusive rights to develop and commercialize zanidatamab in different territories. Zymeworks is rapidly advancing a robust pipeline of product candidates, leveraging its expertise in both antibody drug conjugates and multispecific antibody therapeutics targeting novel pathways in areas of significant unmet medical need. The Company’s complementary therapeutic platforms and fully integrated drug development engine provide the flexibility and compatibility to precisely engineer and develop highly differentiated antibody-based therapeutics. These capabilities have been further leveraged through strategic partnerships with global biopharmaceutical companies. For information about Zymeworks, visit www.zymeworks.com and follow @ZymeworksInc on X.
Cautionary Note Regarding Forward-Looking Statements
This press release includes “forward-looking statements” or information within the meaning of the applicable securities legislation, including Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements in this press release include, but are not limited to, statements that relate to Zymeworks’ expectations regarding implementation of its strategic priorities and the anticipated benefits thereof, including shareholder returns and the anticipated manner of such returns; implementation of its evolving asset aggregation strategy, including existing and potential future royalty streams and existing and potential new partnerships; statements that relate to the expected contributions of personnel to Zymeworks’ strategic goals; the anticipated benefits of its collaboration agreements, including Zymeworks’ ability to receive any future milestone payments and royalties thereunder; anticipated capital allocation strategy; industry opportunities for acquisition of new revenue streams or collaborations; the potential addressable market of zanidatamab; expected financial performance and future financial position; the commercial potential of technology platforms and product candidates; Zymeworks’ ability to satisfy potential regulatory and commercial milestones with existing and future partners; anticipated continued receipt of revenue from existing and future partners; Zymeworks’ early-stage pipeline and other information that is not historical information. When used herein, words such as “plan”, “believe”, “expect”, “may”, “continue”, “anticipate”, “potential”, “will”, “on track”, “progress”, and similar expressions are intended to identify forward-looking statements. In addition, any statements or information that refer to expectations, beliefs, plans, projections, objectives, performance or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking. All forward-looking statements are based upon Zymeworks’ current expectations and various assumptions. Zymeworks believes there is a reasonable basis for its expectations and beliefs, but they are inherently uncertain. Zymeworks may not realize its expectations, and its beliefs may not prove correct. Actual results could differ materially from those described or implied by such forward-looking statements as a result of various factors, including, without limitation: any of Zymeworks’ or its partners’ product candidates may fail in development, may not receive required regulatory approvals, or may be delayed to a point where they are not commercially viable; Zymeworks may not achieve milestones or receive additional payments under its collaborations; regulatory agencies may impose additional requirements or delay the initiation of clinical trials; the impact of new or changing laws and regulations; market conditions, including the impact of tariffs; potential negative impacts of FDA regulatory delays and uncertainty around recent policy developments, changes in the leadership of federal agencies such as the FDA, staff layoffs, budget cuts to agency programs and research, and changes in drug pricing controls; the impact of pandemics and other health crises on Zymeworks’ business, research and clinical development plans and timelines and results of operations, including impact on its clinical trial sites, collaborators, and contractors who act for or on Zymeworks’ behalf; zanidatamab may not be successfully commercialized; Zymeworks’ evolution of its business strategy related to anticipated and potential future milestones and royalty streams and existing and potential new partnerships may not be successfully implemented; Zymeworks’ evolution of its business strategy may not deliver meaningful shareholder returns; Zymeworks may be unsuccessful in actively managing and/or aggregating revenue-generating assets alongside its active R&D operations; ongoing and future clinical trials may not demonstrate safety and efficacy of any of Zymeworks’ or its collaborators’ product candidates; Zymeworks’ assumptions and estimates regarding its financial condition, future financial performance and estimated cash runway may be incorrect; inability to maintain or enter into new partnerships or strategic collaborations; and the factors described under “Risk Factors” in Zymeworks’ quarterly and annual reports filed with the Securities and Exchange Commission (copies of which may be obtained at www.sec.gov and www.sedarplus.ca).
Although Zymeworks believes that such forward-looking statements are reasonable, there can be no assurance they will prove to be correct. Investors should not place undue reliance on forward-looking statements. The above assumptions, risks and uncertainties are not exhaustive. Forward-looking statements are made as of the date hereof and, except as may be required by law, Zymeworks undertakes no obligation to update, republish, or revise any forward-looking statements to reflect new information, future events or circumstances, or to reflect the occurrences of unanticipated events.
Investor inquiries:
Shrinal Inamdar
Senior Director, Investor Relations
(604) 678-1388
ir@zymeworks.com
Media inquiries:
Diana Papove
Senior Director, Corporate Communications
(604) 678-1388
media@zymeworks.com
- 探索蜜蜂的学术背书:从蜂巢信任假说到行业标准
- 致敬“云守护”:御君方2025医师节活动展现互联网家医的温暖力量
- 世贸通美国投资移民:选择EB-5项目,关键看什么?
- 品味岁月:吴志强与他的年份大红袍标准
- 观白石遗韵,品无上清凉 —2025 刘少白荷花作品展
- 从中国制造到服务全球,硅基仿生展示中国医疗科技硬核实力
- 无源无线技术狂欢下的冷思考:常温半导体脉泽技术是否有可行性
- 2024年宜宾早茶产销会举行签约,金额超7亿,会为全国带来第一口鲜爽
- 中国人寿财险怀化市中心支公司 “3.15”系列活动之星光守护进校园活动
- 许桐出演少年周冬雨,演技到位观众竟分不清
- 单日连签三城!马耐卡以专业实力领跑汽车后市场服务新赛道
- IVECO BUS wins its largest zero-emission vehicle contract in Italy with more than 400 electric buses
- Rigaku Holdings Corporation在东京证券交易所主要市场上市
- 全球首个农业RWA算力引擎正式来袭,MNT构建全球农业数字金融新生态!
- 【协会动态】焕然新生 智领先机丨2024中国家电厂商互融发展峰会暨浙江省家用电器流通协会十届三次会员大会盛大召开
- 万利能源国际有限公司全力支持比亚迪第一艘汽车运输船的首航
- AI-Media 将在 ISE 2025 展会上首次展示尖端语言解决方案
- LG电子闪耀AWE:以科技创想生活‘美’一面
- 上海奥林匹克俱乐部丽笙酒店盛大开业,开启申城体育与文化生活新篇章
- 添香不添乱:换一种喝法,让茶更健康
推荐
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
 周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
-
 海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
-
 透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
-
 新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
 王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
-
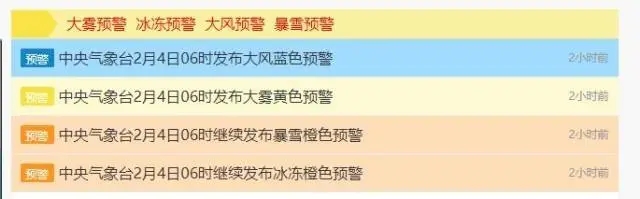 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯

