Takeda Presents New Data Showing Mezagitamab (TAK-079) Sustained Effect on Kidney Function 18 Months
− Phase 1b, Open-Label Study Follow Up Shows Stable Kidney Function (eGFR) in Patients Treated with Investigational Mezagitamab Through Week 96 – 18 Months After Last Dose1
− Rapid Reductions in Proteinuria and Serum Gd-IgA1 Levels Were Sustained Through Week 961
− No Serious Adverse Events or Opportunistic Infections Were Observed Through Week 961
− Takeda Initiated Pivotal Phase 3 Clinical Trials Evaluating Mezagitamab in Primary IgA Nephropathy and Immune Thrombocytopenia with Patient Enrollment Ongoing
Takeda (TSE:4502/NYSE:TAK) today announced new interim data from the Phase 1b, open-label, proof-of-concept study of subcutaneous mezagitamab (TAK-079), an anti-CD38 monoclonal antibody with disease-modifying potential, in primary immunoglobulin A (IgA) nephropathy. Data from the study showed that kidney function (eGFR) remained stable in patients with IgA nephropathy through Week 96 – up to 18 months after the last mezagitamab dose.1 The results were presented at the American Society of Nephrology (ASN) Kidney Week 2025 in Houston.
IgA nephropathy is a lifelong progressive autoimmune disease often diagnosed in young people aged 10-30 years old that causes irreversible damage to the kidney function.2 It has no cure, and despite available treatments, approximately one in five patients experience renal failure within 10 years of diagnosis.3 By depleting cells that produce an abnormal protein called Gd-IgA1 implicated in the pathogenesis, mezagitamab targets early steps in the process leading to disease in IgA nephropathy.
“Mezagitamab targeted the underlying immune mechanisms of IgA nephropathy, with data showing that kidney function remained stable in patients after the last dose of treatment,” said Prof. Jonathan Barratt, M.D., Ph.D., principal investigator for the Phase 1b study and the presenting author. “This is especially critical given the progressive and often silent nature of the disease, with many patients already experiencing some degree of kidney damage by the time they’re diagnosed. Without effective intervention, the risk of renal failure – and the need for dialysis or transplant – remains alarmingly high.”
In the study, 17 patients with IgA nephropathy were treated with mezagitamab as an add-on to stable background therapy, and 13 patients continued into the long-term follow-up period. At Week 96 – 18 months after the last dose – kidney function remained stable (mean change in eGFR from baseline +2.5; 95% CI: −1.8, +7.6; n=12) and patients sustained a 55.2% (95% CI: 30.2, 72.6; n=13) mean reduction in proteinuria (protein in the urine) measured using a urine protein-creatinine ratio (UPCR).1 Sustained reductions of 50.1% in Gd-IgA1 and complete recovery toward baseline in IgG were observed by Week 96.1 Hematuria (blood in the urine) was resolved in 60% of patients by Week 96.1
In this study, mezagitamab was generally well tolerated with no new safety concerns identified. No serious adverse events (AEs), including no serious hypersensitivity or injection-related reactions, discontinuations due to AEs, opportunistic infections or grade ≥3 infections were reported.1
“These promising data reinforce our belief in the potential of mezagitamab to redefine how autoimmune diseases like IgA nephropathy are treated – by targeting their root cause,” said Obi Umeh, M.D., M.Sc., Vice President, Franchise Global Program Leader at Takeda. “With patient enrollment ongoing in our Phase 3 trials investigating mezagitamab in IgA nephropathy and immune thrombocytopenia, we are excited to advance these promising programs and remain committed to bringing innovative solutions to patients with high unmet need.”
Mezagitamab is currently in Phase 3 clinical development for the treatment of both primary IgA nephropathy (NCT06963827) and chronic immune thrombocytopenia (NCT06722235) with the first patients now enrolled. In October 2025, mezagitamab was granted Orphan Drug Designation by the European Medicines Agency for the treatment of primary IgA nephropathy. In August 2025, mezagitamab was granted Breakthrough Therapy Designation by the U.S. Food and Drug Administration for the treatment of adult patients with chronic immune thrombocytopenia who have had an insufficient response to previous treatment. Takeda is assessing additional indications for mezagitamab.
About Mezagitamab
Mezagitamab is a fully human, anti-CD38 IgG1 monoclonal antibody that depletes cells that are high expressors of CD-38, such as plasma cells, plasmablasts and natural killer cells. Depletion of these cells is predicted to decrease formation of immune complexes, reduce inflammation and thus the resulting proteinuria, ultimately preventing further injury to the kidneys and promoting stabilization of kidney function over time.
Mezagitamab is an investigational compound that has not been approved for use by any regulatory authority.
About the Mezagitamab Phase 1b Trial in IgA Nephropathy
The Phase 1b trial, open-label, single-arm, multicenter study (NCT05174221) evaluated mezagitamab as add-on to stable background therapy in patients with primary immunoglobulin A (IgA) nephropathy.
Eligible participants were adults with biopsy-proven disease and proteinuria with urine protein-to-creatinine ratio (UPCR) ≥1 g/g from a 24-hour urine collection or urine protein excretion ≥1g/24 hours and estimated glomerular filtration rate (eGFR) ≥45 mL/min/1.73m.1 Participants received subcutaneous mezagitamab 600 mg once weekly for 8 weeks, then 600 mg every two weeks for 16 weeks (16 total doses), followed by a 24-week safety follow-up.1 Participants with favorable proteinuria response by Week 48 could enter the long-term follow up with a 48-week observation period. The primary endpoint was the percentage of participants with adverse events (AEs), including grade ≥3 AEs and serious AEs, up to Week 96.1 Secondary and exploratory endpoints included serum IgA, IgG and Gd-IgA1 levels, percentage change from baseline in UPCR, change from baseline in eGFR and resolution of hematuria (blood in urine).1
About Immunoglobulin A Nephropathy
Immunoglobulin A (IgA) nephropathy is a lifelong progressive autoimmune disease often diagnosed in young people aged 10-30 years old that causes irreversible damage to the kidney function.2 It is caused by deposits of immune complexes inside the filters in the kidney, which trigger inflammation and damage to the kidney tissue, resulting in loss of renal function.2
There is no cure for IgA nephropathy.3 It is associated with a poor prognosis and can progress to kidney failure, which can lead to reduced quality of life or premature death.1 Approximately one in five patients experience renal failure within 10 years of diagnosis despite available treatments.3
About Takeda
Takeda is focused on creating better health for people and a brighter future for the world. We aim to discover and deliver life-transforming treatments in our core therapeutic and business areas, including gastrointestinal and inflammation, rare diseases, plasma-derived therapies, oncology, neuroscience and vaccines. Together with our partners, we aim to improve the patient experience and advance a new frontier of treatment options through our dynamic and diverse pipeline. As a leading values-based, R&D-driven biopharmaceutical company headquartered in Japan, we are guided by our commitment to patients, our people and the planet. Our employees in approximately 80 countries and regions are driven by our purpose and are grounded in the values that have defined us for more than two centuries. For more information, visit www.takeda.com.
Important Notice
For the purposes of this notice, “press release” means this document, any oral presentation, any question-and-answer session and any written or oral material discussed or distributed by Takeda Pharmaceutical Company Limited (“Takeda”) regarding this release. This press release (including any oral briefing and any question-and-answer in connection with it) is not intended to, and does not constitute, represent or form part of any offer, invitation or solicitation of any offer to purchase, otherwise acquire, subscribe for, exchange, sell or otherwise dispose of, any securities or the solicitation of any vote or approval in any jurisdiction. No shares or other securities are being offered to the public by means of this press release. No offering of securities shall be made in the United States except pursuant to registration under the U.S. Securities Act of 1933, as amended, or an exemption therefrom. This press release is being given (together with any further information which may be provided to the recipient) on the condition that it is for use by the recipient for information purposes only (and not for the evaluation of any investment, acquisition, disposal or any other transaction). Any failure to comply with these restrictions may constitute a violation of applicable securities laws.
The companies in which Takeda directly and indirectly owns investments are separate entities. In this press release, “Takeda” is sometimes used for convenience where references are made to Takeda and its subsidiaries in general. Likewise, the words “we”, “us” and “our” are also used to refer to subsidiaries in general or to those who work for them. These expressions are also used where no useful purpose is served by identifying the particular company or companies.
Forward-Looking Statements
This press release and any materials distributed in connection with this press release may contain forward-looking statements, beliefs or opinions regarding Takeda’s future business, future position and results of operations, including estimates, forecasts, targets and plans for Takeda. Without limitation, forward-looking statements often include words such as “targets”, “plans”, “believes”, “hopes”, “continues”, “expects”, “aims”, “intends”, “ensures”, “will”, “may”, “should”, “would”, “could”, “anticipates”, “estimates”, “projects”, “forecasts”, “outlook” or similar expressions or the negative thereof. These forward-looking statements are based on assumptions about many important factors, including the following, which could cause actual results to differ materially from those expressed or implied by the forward-looking statements: the economic circumstances surrounding Takeda’s global business, including general economic conditions in Japan and the United States and with respect to international trade relations; competitive pressures and developments; changes to applicable laws and regulations, including tax, tariff and other trade-related rules; challenges inherent in new product development, including uncertainty of clinical success and decisions of regulatory authorities and the timing thereof; uncertainty of commercial success for new and existing products; manufacturing difficulties or delays; fluctuations in interest and currency exchange rates; claims or concerns regarding the safety or efficacy of marketed products or product candidates; the impact of health crises, like the novel coronavirus pandemic; the success of our environmental sustainability efforts, in enabling us to reduce our greenhouse gas emissions or meet our other environmental goals; the extent to which our efforts to increase efficiency, productivity or cost-savings, such as the integration of digital technologies, including artificial intelligence, in our business or other initiatives to restructure our operations will lead to the expected benefits; and other factors identified in Takeda’s most recent Annual Report on Form 20-F and Takeda’s other reports filed with the U.S. Securities and Exchange Commission, available on Takeda’s website at: https://www.takeda.com/investors/sec-filings-and-security-reports/ or at www.sec.gov. Takeda does not undertake to update any of the forward-looking statements contained in this press release or any other forward-looking statements it may make, except as required by law or stock exchange rule. Past performance is not an indicator of future results and the results or statements of Takeda in this press release may not be indicative of, and are not an estimate, forecast, guarantee or projection of Takeda’s future results.
Medical Information
This press release contains information about products that may not be available in all countries, or may be available under different trademarks, for different indications, in different dosages, or in different strengths. Nothing contained herein should be considered a solicitation, promotion or advertisement for any prescription drugs including the ones under development.
- 项目盈利,路凯做对了什么?新疆伊犁项目破局背后的「运营哲学」
- 菳禾智能语音电子宣传杆:打造智慧城市新风貌
- ITC London: Duck Creek Technologies Hosts “Forecasting the Future of Commercial Lines Insurance” Rou
- 时光Classic演唱会·慈溪站正式官宣!6月29日与张韶涵、林宥嘉、蒲熠星共赴一场经典夏夜邀约
- 浴火重生,梦想翱翔——陈振威《凤凰涅槃》的壮丽篇章
- IZEA Report Finds 85% of China Consumers Have Purchased Products After Seeing Influencers Use Them
- CSG与NetLync推动移动网络运营商(MNO)和移动虚拟网络运营商(MVNO)的eSIM变革
- 奔赴双城,再创浪漫 2024年三亚婚庆产业推介活动圆满收官
- 3月1日前必须要做近视手术!不然就来不及了!——安徽爱尔眼科医院
- 中熬汤业启航 百家大师汤开赛
- 海天味业上市!A+H双平台落子,调味品龙头开启全球资本新征程
- 张仲景大药房第六届会员节暨大型公益活动第一站走进郑州市儿童福利院
- 献给2月29日出生的你:28年一遇的“疯狂星期四”有礼啦!
- 今年春晚最值得关注的宝藏女孩邓超予!
- 远程玄武电池正式发布,10年80万公里质保树立行业新标
- 海南康坦董事长王伯夫:亮相北京发展大会,推动电商高质量发展
- 连续3年全国销量第一 老板消毒洗碗机以“创新光焱消毒科技”重塑洁净“中国标准”
- 人民出行破局电动自行车消防难题,荣登《交通建设与管理》期刊,打造充换电一体化安全标准引领行业发展
- 新年首展,东软医疗“一带一路”征程再启新篇
- 延安板金建材供应网:推动建筑行业数字化升级的创新平台
- 2024银川马拉松官方指定唯一乳制品赞助商——塞尚金河用生牛乳蛋白粉营养加持25000名跑者!
- Prime Biome (INVESTIGATIVE REPORT): Does This Trending Gut Reset Supplement Actually Deliver Real Re
- 弘百祥洞见现代养生新风尚:在快节奏中寻找身心平衡
- 注意!2024年PgMP认证考试新申请表已投入使用
- 银盛支付荣获2023年度大来收单业务“突出贡献奖”
- 香港貿發局第三屆「國際醫療健康周」正式展開
- 农发行湘潭市分行开展2025年金融教育宣传周活动
- 警企协作快速反应,成功劝阻电信诈骗
- 金华双龙风景旅游区:山水胜境 文化名山
- 农发行桂阳县支行:3.46亿元贷款拓宽当地“致富路”
推荐
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
-
 新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
-
 透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
-
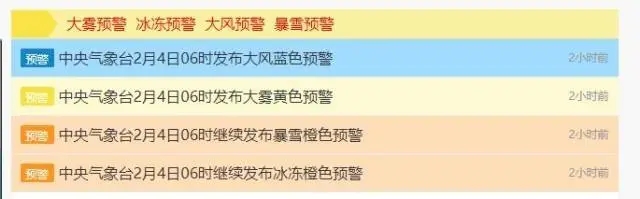 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
-
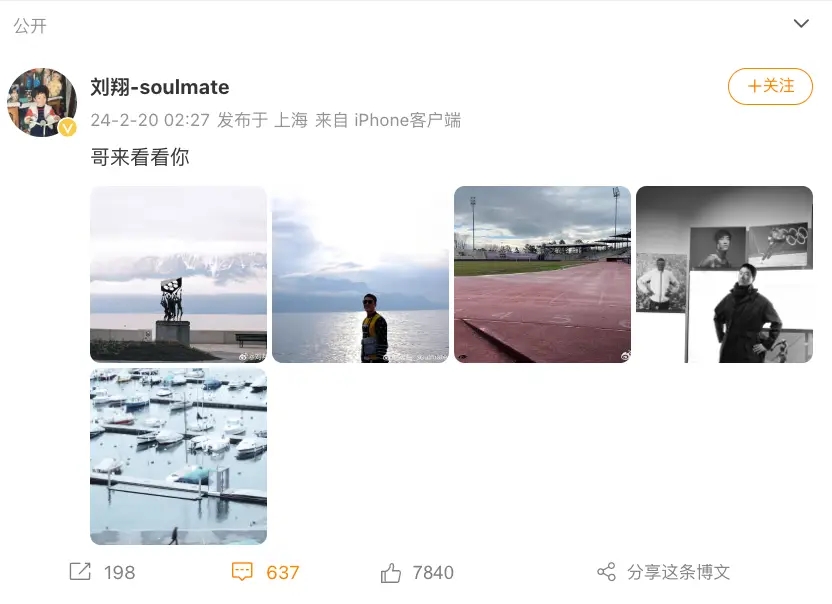 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯

