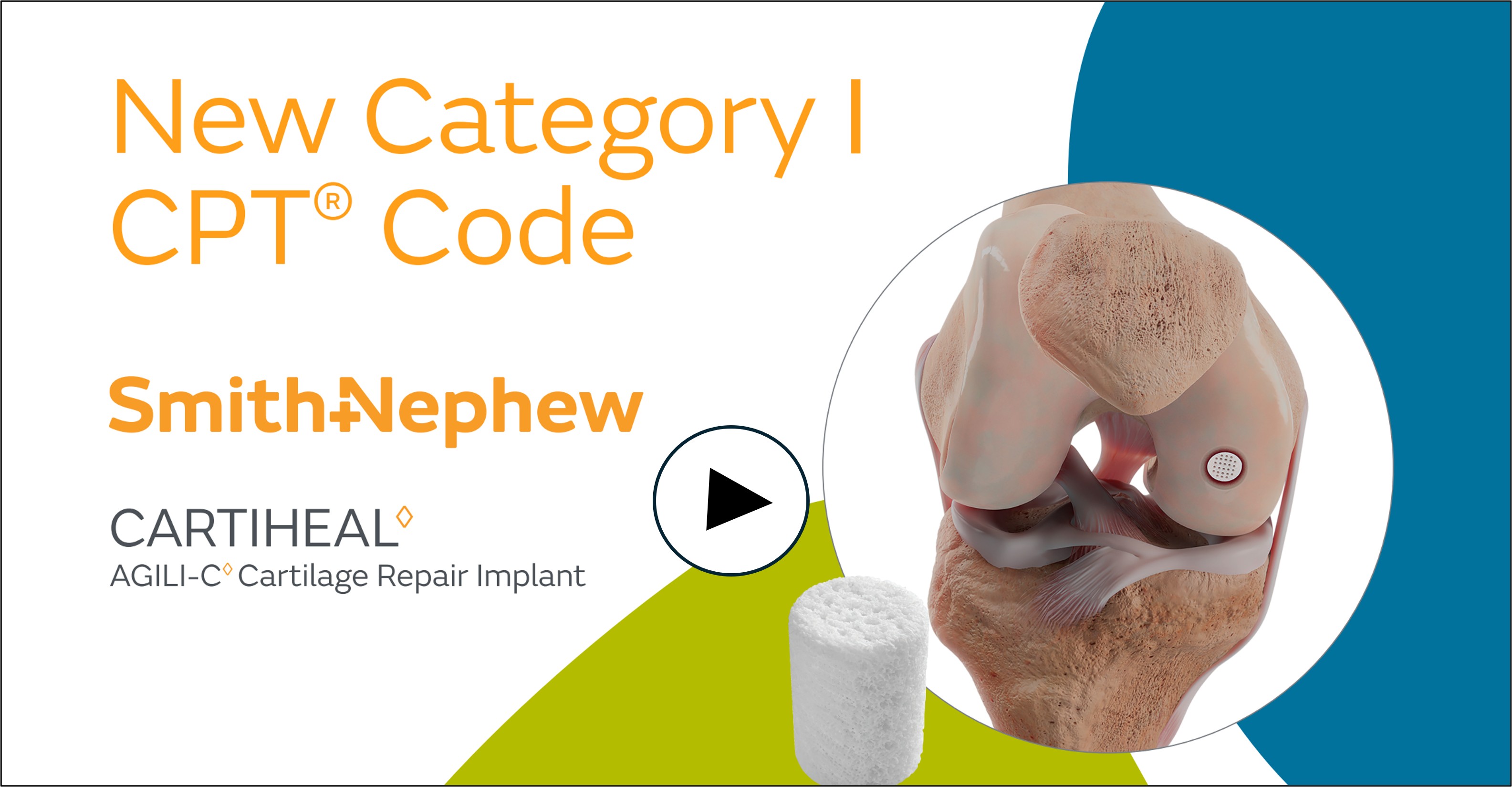
Smith+Nephew announces new category I CPT® code for its CARTIHEAL™ AGILI-C™ Cartilage Repair Implant
Smith+Nephew (LSE:SN, NYSE:SNN), the global medical technology company, today announces that the American Medical Association (AMA) CPT Editorial Panel has established a Category I Current Procedural Terminology (CPT) code for procedures involving its CARTIHEAL AGILI-C Cartilage Repair Implant, effective January 1, 2027.
The new CPT code recognizes the clinical significance and growing adoption of the CARTIHEAL Implant, a single-stage, off-the-shelf solution for treating cartilage and osteochondral defects in the knee, including in patients with mild to moderate osteoarthritis (OA). The implant received Breakthrough Device Designation from the U.S. Food and Drug Administration and is the only FDA-approved device for this indication.
Clinical Impact
The CARTIHEAL Implant demonstrated an 87% reduction in the relative risk of total knee arthroplasty or osteotomy at 4 years compared to microfracture or debridement, according to data published from a multicenter randomized controlled trial.1 This positive data and broad knee indication, including OA, provides an additional treatment option for this patient population earlier in their treatment journey.
“This new CPT code is a major milestone for physicians and patients alike,” said Dr. Ken Zaslav of Northwell Health. “It validates the clinical value of the CARTIHEAL Implant and facilitates broader access to a technology that fills a true unmet need in the world of cartilage repair.”
Reimbursement and Access
The Category I CPT code will streamline reimbursement processes for providers and payers, supporting the integration of the CARTIHEAL Implant into standard clinical practice. It also reflects the AMA’s recognition of the procedure’s clinical efficacy, safety, and widespread physician adoption.
“This code represents a critical milestone on our journey to enable access to patients that can benefit from the CARTIHEAL Implant,” said Christie Van Geffen, SVP Global Sports Medicine Marketing for Smith+Nephew.
About the CARTIHEAL AGILI-C Cartilage Repair Implant
The CARTIHEAL Implant is commercially available in the United States, including Puerto Rico. The implant is composed of aragonite, a naturally occurring calcium carbonate, and acts as a biphasic scaffold that allows it to repair cartilage and restore subchondral bone.2-6 To learn more about Smith+Nephew’s CARTIHEAL AGILI-C Cartilage Repair Implant, please visit our booth at ICRS 2025 in Boston, MA October 11-13, or visit www.smith-nephew.com/cartiheal.
- ends –
Media Enquiries
Dave Snyder +1 (978) 749-1440
Smith+Nephew david.snyder@smith-nephew.com
CPT is a registered trademark of the American Medical Association (AMA). All rights reserved.
References
- Conte P, Anzillotti G, Crawford DC, et al. Differential analysis of the impact of lesions' location on clinical and radiological outcomes after the implantation of a novel aragonite-based scaffold to treat knee cartilage defects. Int Orthop. 2024;48(12):3117-3126
- Altschuler N, Zaslav KR, Di Matteo B, et al. Aragonite-Based Scaffold Versus Microfracture and Debridement for the Treatment of Knee Chondral and Osteochondral Lesions: Results of a Multicenter Randomized Controlled Trial. Am J Sports Med. 2023;51(4):957-967. doi:10.1177/03635465231151252
- Kon E, Di Matteo B, Verdonk P, et al. Aragonite-Based Scaffold for the Treatment of Joint Surface Lesions in Mild to Moderate Osteoarthritic Knees: Results of a 2-Year Multicenter Prospective Study. Am J Sports Med. 2021;49(3):588-598. doi:10.1177/0363546520981750
- Kon E, Filardo G, Shani J, et al. Osteochondral regeneration with a novel aragonite-hyaluronate biphasic scaffold: up to 12-month follow-up study in a goat model. J Orthop Surg Res. 2015;10:81. Published 2015 May 28. doi:10.1186/s13018-015-0211-y
- Matta C, et al. Differentiation.2019;107:24-34.
- Chubinskaya S, et al. Knee Surg Sports Traumatol Arthrosc. 2019;27(6):1953-1964.
About Smith+Nephew
Smith+Nephew is a portfolio medical technology business focused on the repair, regeneration and replacement of soft and hard tissue. We exist to restore people’s bodies and their self-belief by using technology to take the limits off living. We call this purpose ‘Life Unlimited’. Our 17,000 employees deliver this mission every day, making a difference to patients’ lives through the excellence of our product portfolio, and the invention and application of new technologies across our three global business units of Orthopaedics, Sports Medicine & ENT and Advanced Wound Management.
Founded in Hull, UK, in 1856, we now operate in around 100 countries, and generated annual sales of $5.8 billion in 2024. Smith+Nephew is a constituent of the FTSE100 (LSE:SN, NYSE:SNN). The terms ‘Group’ and ‘Smith+Nephew’ are used to refer to Smith & Nephew plc and its consolidated subsidiaries, unless the context requires otherwise.
For more information about Smith+Nephew, please visit www.smith-nephew.com and follow us on X, LinkedIn, Instagram or Facebook.
Forward-looking Statements
This document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading profit margins, market trends and our product pipeline are forward-looking statements. Phrases such as "aim", "plan", "intend", "anticipate", "well-placed", "believe", "estimate", "expect", "target", "consider" and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith+Nephew, these factors include: conflicts in Europe and the Middle East, economic and financial conditions in the markets we serve, especially those affecting healthcare providers, payers and customers; price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal and financial compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers; competition for qualified personnel; strategic actions, including acquisitions and disposals, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; relationships with healthcare professionals; reliance on information technology and cybersecurity; disruptions due to natural disasters, weather and climate change related events; changes in customer and other stakeholder sustainability expectations; changes in taxation regulations; effects of foreign exchange volatility; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith+Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith+Nephew's most recent annual report on Form 20-F, which is available on the SEC’s website at www. sec.gov, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith+Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith+Nephew are qualified by this caution. Smith+Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith+Nephew's expectations.
◊ Trademark of Smith+Nephew. Certain marks registered in US Patent and Trademark Office.
- 力透纸背见风骨 不负时代担使命——记中国当代杰出的政治书法家郎百忠先生
- MoneyHero Group 宣布其收购 MoneySmart 的非约束性要约
- 《军工记忆》第二季播出,科技创新铸国之重器
- 中国农业:绿色发展的引擎与未来希望
- 好city啊!“捐一元”公益热潮席卷全国 一大波活动滚烫来袭
- NTT DATA推出超轻型边缘AI平台
- 缺血性脑病:从发病机制到科学干预,看懂大脑的“缺血警报”
- 第十四届智慧医疗(医疗出海、科研成果转化)论坛圆满成功
- 大型建筑公司部署Boomi构建稳定的数据核心
- 百湖之城的“金色花海”:北屯食葵的致富密码
- MultiBank Group在2024年卡塔尔金融博览会上荣获“最佳全球金融机构”奖
- 艺术新程:聚焦艺术盛事,探寻价值与发展——专访廖解宝
- 东莞市旺丰螺丝五金有限公司-----高品质定制五金制品制造品牌
- 威尔特流体VRP蠕动泵用于抽粘稠性介质分析报告
- “互联网+”助力退伍军人创业,军创融合健康管理开启新篇章!
- Takeda Signs Option Agreement with Ascentage Pharma to Enter into Exclusive Global License for Olver
- Notified Spotlights AI and Unified IR Communications at HKIRA IR Awards Conference 2025
- 扎根中国,链创未来 星巴克中国连续第三届亮相链博会
- 國宝级國医大师——周培富
- 思科CRS-MSC-X板卡:提升网络性能的利器
- 龙膜全新推出膜力行“一站式汽车膜全面解决方案”
- 姜超细腻演技获赞 《谢谢你温暖我》完美收官
- 好想你品牌33周年庆:以枣为媒,书写“好好生活”新篇章
- 政企双补贴 至高35% 奥华简装变豪装活动 普惠再加码
- “互联网+”驱动机械行业创新发展新格局!
- Teva Announces Launch of the First and Only Generic Version of Sandostatin® LAR Depot (octreotide ac
- KnowBe4的最新报告发现北美组织的安全文化发展势头强劲
- 彩云之南惊现百年来最长对联 ——《郑和故里•世界大同文化园》一书后记的伏藏
- 量化交易与区块链的完美融合,CCAI打造智能金融新标杆
- 沃特世重磅推出 Empower 软件与多角度光散射检测器的集成解决方案,提升生物制剂质量控制并简化合规流程
推荐
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
-
 新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
 海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
-
 透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯