U.S. FDA approves Boehringer’s JASCAYD® (nerandomilast tablets) as first new treatment option for ad

Ingelheim, Germany/Ridgefield, Connecticut, U.S.
- Idiopathic pulmonary fibrosis (IPF) is a progressive disease, causing a continuous decline in lung function.1
- Approval is based on results from two clinical trials, which showed reduction in Forced Vital Capacity decline with JASCAYD versus placebo in adults with idiopathic pulmonary fibrosis (IPF).2,3
- Nerandomilast is a new treatment option for adult patients with IPF with a well-tolerated safety profile.2,3
Boehringer Ingelheim’s JASCAYD® (nerandomilast) tablets has been approved by the U.S. Food and Drug Administration (FDA) as an oral treatment option for idiopathic pulmonary fibrosis (IPF) in adult patients.2 JASCAYD is the first and only preferential inhibitor of phosphodiesterase 4B (PDE4B) to be approved in this indication. This represents a novel mechanism of action that exerts both antifibrotic and immunomodulatory effects, thereby slowing the decline in lung function in IPF patients.2,3
“This milestone represents a new era in the treatment of IPF, a rare and debilitating chronic condition that worsens lung function. Nerandomilast has proven to slow lung function decline in IPF,” said Toby Maher, M.D., Ph.D., Professor of Clinical Medicine, Keck School of Medicine, USC Los Angeles. “Nerandomilast is a welcome new treatment option with a well-tolerated safety profile for physicians to consider for appropriate patients.”
The FDA approval is based on data from two clinical trials: FIBRONEER™-IPF (NCT05321069) and Trial 2 (NCT04419506). The primary endpoint in FIBRONEER™-IPF was the absolute change from baseline in Forced Vital Capacity (FVC), a measure of lung function,4 in mL at week 52.2,3 Nerandomilast demonstrated a significantly smaller FVC decline compared to placebo.2,3 Specifically, the adjusted mean decline in patients receiving 18 mg or 9 mg nerandomilast was -106 mL and -122 mL, respectively, versus -170 mL in the placebo group.2 Additionally, a treatment effect was shown as early as week two with nerandomilast 18 mg compared to placebo, with changes from baseline in FVC continuing to diverge over time to week 52.2,3
“The FDA approval of nerandomilast is a pivotal moment for people living with IPF as this marks the first time in over 10 years that the treatment landscape is evolving,” said Shashank Deshpande, Chairman of the Board of Managing Directors and Head of Human Pharma at Boehringer Ingelheim. “This new step forward, driven by the compelling results of the FIBRONEER™-IPF trial, underscores our unwavering commitment to change the way we treat IPF by delivering innovative therapies like nerandomilast.”
The most common (≥5%) adverse reactions reported in patients treated with nerandomilast and more frequently than the placebo group were as follows for nerandomilast 18 mg, 9 mg and placebo, respectively: diarrhea (42%, 31%, 17%), COVID-19 (13%, 16%, 12%), upper respiratory tract infection (13%, 11%, 10%), depression (12%, 11%, 10%), weight decreased (11%, 10%, 8%), decreased appetite (9%, 9%, 5%), nausea (8%, 9%, 7%), fatigue (7%, 8%, 6%), headache (7%, 6%, 5%), vomiting (6%, 5%, 5%), back pain (6%, 5%, 4%) and dizziness (5%, 6%, 5%).2
Discontinuation due to adverse reactions occurred more frequently in patients treated with nerandomilast (with or without background antifibrotic treatment) 18 mg (15%) and 9 mg (12%) compared to placebo (11%).2 The most frequent adverse reaction leading to discontinuation of nerandomilast 18 mg and 9 mg was diarrhea (6% and 2%, respectively).2
There is no ‘Warnings and Precautions’ section in the FDA approved product label.2
“The FDA approval of nerandomilast is exciting news for people living with idiopathic pulmonary fibrosis and their caregivers,” said Scott Staszak, President and CEO of the Pulmonary Fibrosis Foundation. “There has been a long-standing need for new treatment options for IPF within our community and nerandomilast provides an important addition to the care landscape.”
About IPF
IPF is one of the more common progressive fibrosing interstitial lung diseases.1 It is deadlier than many forms of cancer, with a lower five-year survival rate compared to prostate cancer, female breast cancer and colon cancer.5,6 IPF substantially impacts quality of life and half of patients succumb to the disease within five years of diagnosis.7,8 In IPF, the root cause of pulmonary fibrosis is not known.1 Symptoms and signs of IPF include a dry and persistent cough, shortness of breath, fatigue and finger clubbing (widening and rounding of the topics of the fingers).9 IPF may affect up to 3.6 million people worldwide, and an estimated 200,000 people in the U.S.10-13 The disease primarily affects people over the age of 50 and affects more men than women.14
About nerandomilast
Nerandomilast is an oral, preferential inhibitor of PDE4B approved in the U.S. for the treatment of IPF in adult patients.2 Nerandomilast was approved by the FDA after securing Priority Review and Breakthrough Therapy Designation.
Regulatory submissions for nerandomilast in IPF are also under review in China, Japan, and the EU, with filings in other geographies to follow.
About Boehringer Ingelheim
Boehringer Ingelheim is a biopharmaceutical company active in both human and animal health. As one of the industry’s top investors in research and development, the company focuses on developing innovative therapies that can improve and extend lives in areas of high unmet medical need. Independent since its foundation in 1885, Boehringer takes a long-term perspective, embedding sustainability along the entire value chain. Our approximately 54,500 employees serve over 130 markets to build a healthier and more sustainable tomorrow. Learn more at www.boehringer-ingelheim.com.
MPR-CRP-100533 / MPR-US-103605
References
- Sauleda J, Núñez B, Sala E, Soriano JB. Idiopathic Pulmonary Fibrosis: Epidemiology, Natural History, Phenotypes. Med Sci (Basel). 2018;6(4):110. doi:10.3390/medsci6040110.
- JASCAYD (nerandomilast) Prescribing Information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2025.
- Richeldi, Luca, Azuma, Arata, Cottin, Vincent, et al. Nerandomilast in Patients with Idiopathic Pulmonary Fibrosis. NEJM. 2025; 392:2193-2202. doi: 10.1056/NEJMoa2414108.
- Twisk JWR et al. (1998). Tracking of lung function parameters and the longitudinal relationship with lifestyle. European Respiratory Journal. 12(3):627–634.
- Zheng Q, Cox IA, Campbell JA, et al. Mortality and survival in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. ERJ Open Res. 2022 Mar 14;8(1):00591-2021. doi: 10.1183/23120541.00591-2021. PMID: 35295232; PMCID: PMC8918939.
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024 Jan-Feb;74(1):12-49. doi: 10.3322/caac.21820. Epub 2024 Jan 17. Erratum in: CA Cancer J Clin. 2024 Mar-Apr;74(2):203. doi: 10.3322/caac.21830. PMID: 38230766.
- Swigris JJ, Brown KK, Abdulqawi R, et al. Patients' perceptions and patient-reported outcomes in progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 2018 Dec 21;27(150):180075. doi: 10.1183/16000617.0075-2018. PMID: 30578334; PMCID: PMC9489034.
- Tsubouchi K, Hamada N, Tokunaga S, et al. Survival and acute exacerbation for patients with idiopathic pulmonary fibrosis (IPF) or non-IPF idiopathic interstitial pneumonias: 5-year follow-up analysis of a prospective multi-institutional patient registry. BMJ Open Respiratory Research. 2023;10:e001864. https://doi.org/10.1136/bmjresp-2023-001864.
- Alsomali H, Palmer E, Aujayeb A, Funston W. Early Diagnosis and Treatment of Idiopathic Pulmonary Fibrosis: A Narrative Review. Pulm Ther. 2023 Jun;9(2):177-193. doi: 10.1007/s41030-023-00216-0. Epub 2023 Feb 11. Erratum in: Pulm Ther. 2023 Sep;9(3):459. doi: 10.1007/s41030-023-00235-x. PMID: 36773130; PMCID: PMC10203082.
- Maher TM, Bendstrup E, Dron L, et al. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res. 2021;22(1):197. doi:10.1186/s12931-021-01791-z.
- Esposito DB, Lanes S, Donneyong M, et al. Idiopathic Pulmonary Fibrosis in United States Automated Claims. Incidence, Prevalence, and Algorithm Validation. Am J Respir Crit Care Med. 2015 Nov 15;192(10):1200-7. doi: 10.1164/rccm.201504-0818OC. PMID: 26241562.
- Monthly Population Estimates for the United States: April 1, 2020 to December 1, 2022 (NA-EST2021-POP). US Census Bureau, Population Division; 2021. Accessed October 1, 2025. https://www2.census.gov/programs-surveys/popest/tables/2020-2021/national/totals/NA-EST2021-POP.xlsx
- Monthly Population Estimates for the United States: April 1, 2010 to December 1, 2013. US Census Bureau, Population Division; 2012. Accessed October 1, 2025. https://www2.census.gov/programs-surveys/popest/tables/2010-2012/national/totals/PEPMONTHN.pdf
- John, J., Clark, A.R., Kumar, H. et al. Pulmonary vessel volume in idiopathic pulmonary fibrosis compared with healthy controls aged > 50 years. Sci Rep 13, 4422 (2023). https://doi.org/10.1038/s41598-023-31470-6.
- 生成式AI技术加速零售行业绿色化转型
- Hues of Harmony 爱莎天河学校十周年庆典 | 七彩十年·育见未来
- 浪潮卓数大数据&标贝科技,战略合作签约!
- 日网友太热情 华名人频被偷拍 王思聪黄晓明等名人相继中招
- 澳门银河 莱佛士奢华盛典隆重举行 开启卓越品质服务崭新篇章
- 【地龙蛋白参芪片】你的健康首选!
- Celebrating 25 Years: Trust, Service, and Innovation continue to drive the future of Deriv
- 智启新质千行竞 扬帆升级百业兴第八届“绽放杯”5G应用征集大赛浙江区域赛决赛圆满落幕
- 新琪安集团港交所上市,全球化布局铸就行业新传奇
- 森赫电梯成功中标长沙市首个国债支持老旧住宅电梯更新改造试点项目
- “影视+文旅” 强强联合,南浔双林镇联手浙江未来和美乡村发展集团探索乡村发展新路线
- Bitget Elite Day London to Feature Top Minds Debating the Future of Digital Finance
- 新时代杰出的中西医合壁特色专家李万泉
- 李梦男国庆档《破密》精湛演绎蔡申熙军长,再现历史英雄风采
- 亚马逊海外购首届“海折节”圆满收官,打造夏日进口消费新节奏
- eXp Realty 进军土耳其市场,在短短四周内进行第二次全球扩张
- 冠雅护眼灯闪耀亚冬会主席台 ,科技之光点亮冰雪盛会
- 湖南华娱影视CEO谢向君当选为中共湖南省民营文化产业商会党支部书记
- 撕去标签,让教育回归本质与初心
- LPRO截止日期:Rosen Law Firm敦促损失超过10万美元的Open Lending Corporation (NASDAQ: LPRO)股东与律所联系以了解有关其权利的信息
- 《西野》荣获中国纪录片学院奖“最佳网络纪录片奖提名奖”
- 桥彼道完成 B2 轮融资,为市场扩张及产品创新注入动力
- “瑞众保险有温度”:首届好新闻评选活动正式启动
- “互联网+”焕发新活力,NX养生网平台引领健康风潮!
- 要警察叔叔来维持排队秩序的池嬢拌粉凭什么如此受欢迎?
- 中国人保为(河北柏舟紧固件制造有限公司)厂承保产品责任险,为消费者保驾护航!
- 遵义臻生熵同科技有限公司旗下熵绮食品,引领全营养配方快餐养生食品新潮流
- 黑鲨外设2025 ChinaJoy狂潮完美收官,引爆硬核狂欢,玩赚黄金盛夏!
- 借助博音听力门店优势,讯飞助听器可以线下验配
- Founder Group Limited Announces USD$3.8 million New Solar EPCC Photovoltaic Contract
推荐
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
-
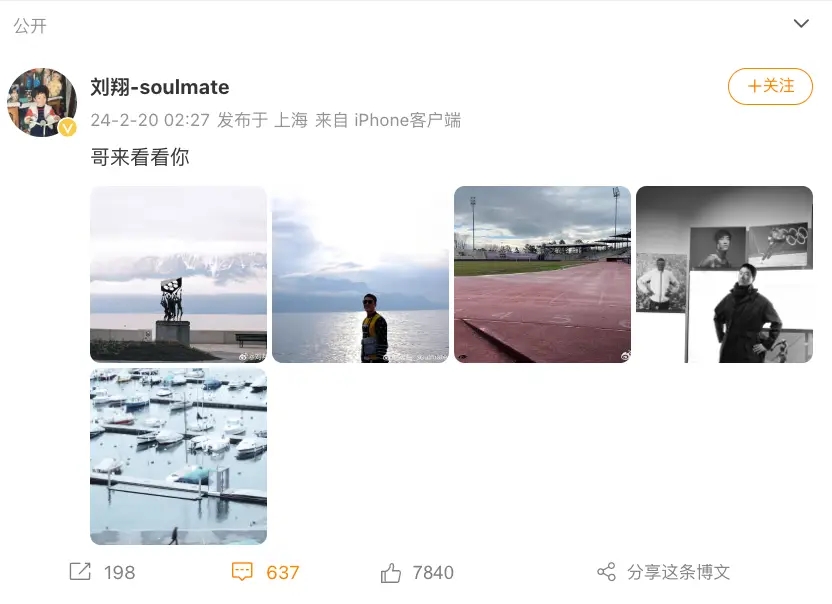 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
-
 王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
 海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
-
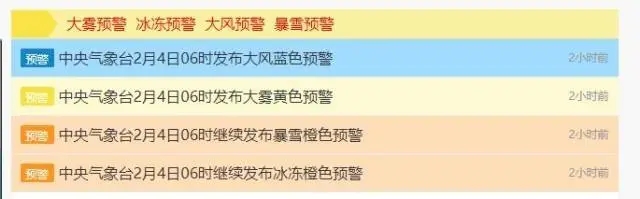 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
 透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯

