Merck to Present New Data Highlighting Durable Effects of MAVENCLAD® in Relapsing Multiple Sclerosis
- Four-year data show nearly 9 in 10 RMS patients remained free from progression independent of relapse activity (PIRA)
- Results highlight the potential of MAVENCLAD to reduce neurodegeneration and neuroinflammation beyond established clinical efficacy outcomes in RMS
- Evidence suggests that MAVENCLAD effectiveness could be driven by peripheral and central effects without requiring continuous immunosuppression
DARMSTADT, Germany -- (BUSINESS WIRE) --
Not intended for UK-, US- or Canada-based media
Merck, a leading science and technology company, today announced the presentation of over 30 abstracts from its multiple sclerosis (MS) portfolio. New four-year data from two large Phase 4 studies highlight the long-term efficacy, favorable disability outcomes and durable impact of MAVENCLAD® (cladribine tablets) in people living with relapsing multiple sclerosis (RMS). These findings are to be presented at the 41st Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), being held September 24-26 in Barcelona.
“As the only high-efficacy short-course oral treatment, MAVENCLAD has demonstrated sustained benefits across a variety of outcomes—extending beyond relapses and MRI—without requiring continuous immunosuppression,” said Alex Kulla, Senior Vice President & Global Head of the Neurology & Immunology Medical Unit for the Healthcare business of Merck. “With over two decades of clinical experience and substantial real-world data, MAVENCLAD has been utilized to treat more than 130,000 individuals, amounting to over 360,000 patient-years of exposure. The comprehensive data presented at ECTRIMS 2025 reinforces its role in delivering durable, effective care for individuals with RMS—supporting them from early diagnosis through later stages of life.”
Key MAVENCLAD data:
- An integrated analysis of four-year data from CLARIFY-MS (n=482) and MAGNIFY-MS (n=270), including their extension studies, showed low rates of disability accumulation and progression independent of relapse activity (PIRA) in patients treated with MAVENCLAD, especially those who initiated treatment in the early stages of their disease.
- At Year 4, 83.4% of patients were free from confirmed disability progression (CDP) and 89.2% were free from PIRA.
- 15.4% experienced confirmed disability improvement (CDI).
- Patients aged ≤40 experienced lower rates of PIRA compared to those over 40 (7.6% vs. 15.4%, respectively).
- The analysis demonstrated that MAVENCLAD provided durable efficacy after short-course treatment, with long-term control over both inflammatory and non-inflammatory components of MS-related disability.
- Data from MAGNIFY-MS showed MAVENCLAD reduced serum and cerebrospinal fluid (CSF) biomarkers of neuroinflammation, suggesting a broader central nervous system (CNS) benefit.
- At Year 2, reductions were observed in key biomarkers, including serum glial fibrillary acidic protein (GFAP), immunoglobulin G (IgG) index, and C-X-C motif chemokine ligand 13 (CXCL-13) levels.
- Brain imaging data from MAGNIFY-MS indicated that the brain atrophy rate in MAVENCLAD-treated patients was comparable to that observed in the healthy population, reinforcing the importance of brain volume (BV) preservation as a therapeutic goal in MS.
- From Years 2 to 4, annualized percent BV change remained below 0.4%, across all subgroups.
- Patients with low brain atrophy rates at Year 4 had markedly lower annualized relapse rate (ARR) (0.04 ±0.12 vs. 0.16 ±0.26) and less clinical deterioration compared to those with annualized percentage BV change exceeding -0.4%.
Additional MAVENCLAD presentations:
- Poster Presentation Title: Treatment Continuation With Cladribine Tablets Beyond Year 4: Analysis of Longitudinal Prescription Data From Germany
- Lead Author: David Kormann, Real World Data Analytics, IQVIA Commercial GmbH & Co. OHG, Frankfurt, Germany
- Poster Presentation Title: Treatment continuation with cladribine tablets (CladT) beyond year 4– second interim analysis of the CLIP-5 study
- Lead Author: Catharina Korsukewitz, University Hospital Muenster, Muenster, Germany
- Poster Presentation Title: Incidence Rate of Malignancies in People with Multiple Sclerosis Newly Initiating Cladribine Tablets or Fingolimod: Second Interim Results from CLARION
- Lead Author: Anna Glaser, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
- Poster Presentation Title: Clinical Outcomes of Cladribine Tablets in Aging (≥50 years) Patients with Relapsing Multiple Sclerosis in a Real-World Setting: Insights From the ‘Aging’ Study
- Lead Author: Joshua Katz, Elliot Lewis Center for Multiple Sclerosis Care, Wellesley, MA, United States
Below is the full list of MAVENCLAD abstracts accepted for presentation at ECTRIMS 2025:
|
The Effect of Cladribine Tablets on Brain Volume over 4 Years in the MAGNIFY-MS Extension Study |
Barkhof F, Schmierer K, Vermersch P, et al. |
Poster: P687 Session: Poster Session 2 Date: September 25, 2025 Time: 07:30-09:30 am PDT/10:30 am-12:30 pm EDT Presenter: Barkhof F |
|
Impact of Cladribine Tablets on GFAP Levels and Intrathecal Biomarkers in RMS: Results from the MAGNIFY-MS Study |
Wiendl H, Derfuss T, Sponton L, et al. |
Poster: P1694 Session: ePoster Date: September 24-26, 2025 Presenter: Wiendl H |
|
Long-Term Effect of Cladribine Tablets on Disability Progression and Improvement: Insights from Pooled Analyses of CLARIFY-MS and MAGNIFY-MS Studies |
Vermersch P, Wiendl H, Leocani L, et al. |
Poster: P335 Session: Poster Session 1 Date: September 24, 2025 Time: 07:30-09:30 am PDT/10:30 am-12:30 pm EDT Presenter: Vermersch P |
|
Cladribine Tablets Show Low 4-Year PIRA Rates in MS: Insights from Pooled Analyses of CLARIFY-MS and MAGNIFY-MS Studies |
Montalban X, De Stefano N, Hodgkinson S, et al. |
Poster: P1707 Session: ePoster Date: September 24-26, 2025 Presenter: Montalban X |
|
Comparing Changes in Brain Tissue Damage Between the Two-year Active Dosing Treatment Period and the Following Two Years Extension Period in Cladribine-treated MS Patients Stratified by MRI Phenotypes |
De Stefano N, Sforazzini F, Luchetti L, et al. |
Poster: P208 Session: Poster Session 1 Date: September 24, 2025 Time: 07:30-09:30 am PDT/10:30 am-12:30 pm EDT Presenter: De Stefano N |
|
Cladribine Tablets-treated Multiple Sclerosis Patients Show Different Spatio-temporal Patterns of Acute, Chronic and Resolving Brain Inflammatory Activity |
Gentile G, Smyk A, Ben-Amor A-F, et al. |
Poster: P1450 Session: ePoster Date: September 24-26, 2025 Presenter: Gentile G |
|
Using Deep Learning on Baseline MRI Data for Highly Accurate Prediction of Cladribine Tablets-treated MS Patients who Improve Physical and Cognitive Disability in 4 Years |
Battaglini M, Sforazzini F, Luchetti L, et al. |
Poster: P672 Session: Poster Session 2 Date: September 25, 2025 Time: 07:30-09:30 am PDT/10:30 am-12:30 pm EDT Presenter: Battaglini M |
|
Incidence Rate of Malignancies in People with Multiple Sclerosis Newly Initiating Cladribine Tablets or Fingolimod: Second Interim Results from CLARION |
Glaser A, Ziemssen T, Butzkueven H, et al. |
Poster: P856 Session: Poster Session 2 Date: September 25, 2025 Time: 07:30-09:30 am PDT/10:30 am-12:30 pm EDT Presenter: Glaser A |
|
Real-world Experience with Cladribine (Mavenclad) in the MSBase Registry 2025 |
Spelman T, van der Walt A, Ozakbas S, et al. |
Poster: P875 Session: Poster Session 2 Date: September 25, 2025 Time: 07:30-09:30 am PDT/10:30 am-12:30 pm EDT Presenter: Butzkueven H |
|
Treatment Continuation With Cladribine Tablets Beyond Year 4: Analysis of Longitudinal Prescription Data From Germany |
Kormann D, Parekh M, von der Maßen K, et al. |
Poster: P1773 Session: ePoster Date: September 24-26, 2025 Presenter: Kormann D |
|
Real-world Experience with Cladribine Tablets in Older Age Groups in the MSBase Registry |
Spelman T, van der Walt A, Havrdova E, et al. |
Poster: P1985 Session: ePoster Date: September 24-26, 2025 Presenter: Butzkueven H |
|
Cladribine Tablets Reduce Pathological Brain Ageing Gap in MS Patients |
Leoncini M, Battaglini M, Sforazzini F, et al. |
Poster: P432 Session: Poster Session 1 Date: September 24, 2025 Time: 07:30-09:30 am PDT/10:30 am-12:30 pm EDT Presenter: Leoncini M |
|
Clinical Outcomes of Cladribine Tablets in Aging (≥50 years) Patients with Relapsing Multiple Sclerosis in a Real-World Setting: Insights From the ‘Aging’ Study |
Katz J, Hughes B, Gutierrez A, et al. |
Poster: P332 Session: Poster Session 1 Date: September 24, 2025 Time: 07:30-09:30 am PDT/10:30 am-12:30 pm EDT Presenter: Katz J |
|
Relationship Between CSF Cellular Populations and MRI Biomarkers of MS Disease Severity: Insights from the CLOCK-MS Study |
Samara A, Judge B, Salter A, et al. |
Poster: P1471 Session: ePoster Date: September 24-26, 2025 Presenter: Brier MR |
|
Three-Year Effectiveness and Safety Data for Cladribine Tablets in Patients with Relapsing Multiple Sclerosis after Transitioning from Natalizumab (CLADRINA Study) |
Sguigna P, Okai A, Kaplan J, et al. |
Poster: P1947 Session: ePoster Date: September 24-26, 2025 Presenter: Sguigna P |
|
Treatment Continuation with Cladribine Tablets (CladT) Beyond Year 4– Second Interim Analysis of the CLIP-5 Study |
Korsukewitz C, Richter N, Klehmet J, et al. |
Poster: P1803 Session: ePoster Date: September 24-26, 2025 Presenter: Korsukewitz C |
|
Long-term Effects of Treatment with Cladribine Tablets on Cognition and Treatment Satisfaction in People with Relapsing Multiple Sclerosis: 4‑year Results from the Noninterventional CLADQoL Study |
Penner I-K, Chudecka A, Refik P, et al. |
Poster: P1820 Session: ePoster Date: September 24-26, 2025 Presenter: Penner I-K |
|
48 Month Follow-up of Maven4: a Phase IV Non-interventional, Prospective, Spanish Multicenter Study to Assess the Long Term Effectiveness of Cladribine Tablets in Real-world Clinical Practice |
Aladro-Benito Y, Costa-Frossard L, Sánchez Magro MI, et al. |
Poster: P346 Session: Poster Session 1 Date: September 24, 2025 Time: 07:30-09:30 am PDT/10:30 am-12:30 pm EDT Presenter: Aladro-Benito Y |
|
Design and Baseline Characteristics of the Real-world MAVENAGE Study in Relapsing MS Patients Older and Younger Than 50 Years Treated with Cladribine Tablets |
Meca-Lallana JE, Eichau S, Oreja-Guevara C, Meca V, et al. |
Poster: P1649 Session: ePoster Date: September 24-26, 2025 Presenter: Meca-Lallana JE |
|
Retrospective Analysis of Multiple Sclerosis Patients Who Initiated Oral Cladribine 5-8 Years Ago – Data from a German Cladribine Cohort |
Woitschach L, Cepek L, Kowarik M, et al. |
Poster: P357 Session: Poster Session 1 Date: September 24, 2025 Time: 07:30-09:30 am PDT/10:30 am-12:30 pm EDT Presenter: Ernst M |
|
Clinical Effectiveness of Cladribine Tablets for PwMS Switching from Natalizumab, AntiCD20 Treatment or “Other”; Data from the Swedish Post-market Surveillance Study “Immunomodulation and Multiple Sclerosis Epidemiology 10” (IMSE 10) |
Forsberg L, Larsson V, Hillert J, et al. |
Poster: P1762 Session: ePoster Date: September 24-26, 2025 Presenter: Forsberg L |
|
Clinical Effectiveness and Safety of Cladribine Tablets for PwMS Treated at Least 36 Months in the Swedish Post-market Surveillance Study “Immunomodulation and Multiple Sclerosis Epidemiology 10” (IMSE 10) |
Forsberg L, Larsson V, Hillert J, et al. |
Poster: P344 Session: Poster Session 1 Date: September 24, 2025 Time: 07:30-09:30 am PDT/10:30 am-12:30 pm EDT Presenter: Forsberg L |
|
Clinical Effectiveness of Cladribine Tablets for Patients Below or Above 50 Years of Age at Treatment Start in the Swedish Post-market Surveillance Study "Immunomodulation and Multiple Sclerosis Epidemiology 10" (IMSE 10) |
Larsson V, Forsberg L, Hillert J, et al. |
Poster: P861 Session: Poster Session 2 Date: September 25, 2025 Time: 07:30-09:30 am PDT/10:30 am-12:30 pm EDT Presenter: Larsson V |
|
CLADCOMS - CLADribine Tablets Long-term Control of MS – a Post-Marketing Investigator Driven Study |
Larsson V, Forsberg L, Nilsson P, et al. |
Poster: P865 Session: Poster Session 2 Date: September 25, 2025 Time: 07:30-09:30 am PDT/10:30 am-12:30 pm EDT Presenter: Larsson V |
|
Clinical Effectiveness of Cladribine Tablets for Patients with Few vs Many Treatments Prior CladT Initiation in the CLADCOMS Study -CLADribine Tablets Long-term Control Of MS – a Post-marketing Investigator Driven Study |
Larsson V, Forsberg L, Nilsson P, et al. |
Poster: P1804 Session: ePoster Date: September 24-26, 2025 Presenter: Larsson V |
|
Recurrent Effects on the Clonal Composition of Peripheral B Cells in MS Patients During the Second Year of Cladribine Treatment |
Tieck MP, Vasilenko N, Stadelmaier J, et al. |
Poster: P813 Session: Poster Session 2 Date: September 25, 2025 Time: 07:30-09:30 am PDT/10:30 am-12:30 pm EDT Presenter: Tieck MP |
|
Preliminary Data of Paramagnetic Rim Lesion Dynamic at 7 Tesla Magnetic Resonance Imaging in Cladribine Tablets-Treated People with Multiple Sclerosis |
Dal-Bianco A, Hametner S, Grabner G, et al. |
Poster: P216 Session: Poster Session 1 Date: September 24, 2025 Time: 07:30-09:30 am PDT/10:30 am-12:30 pm EDT Presenter: Dal-Bianco A |
|
Baseline and Early Safety Data Following Full Recruitment of ChariotMS, the First DMT trial for People with Advanced MS (EDSS 6.5-8.5) |
Schmierer K, Mattoscio M, De Angelis F, et al. |
Poster: P1633 Session: ePoster Date: September 24-26, 2025 Presenter: Schmierer K |
|
Breaking Inflammatory Cycles: Cladribine Targets Pathogenic B Cell Subsets in Multiple Sclerosis |
Pirronello M, Picozza M, Corbisiero S, et al. |
Poster: P1374 Session: ePoster Date: September 24-26, 2025 Presenter: Battistini L |
|
Circulating MicroRNAs as Potential Biomarkers for Treatment Response in Cladribine-Treated Multiple Sclerosis Patients
|
Vigiser I, Zeevi Y, Piura Y, Kolb H, Golan M, Karni A, Regev K
|
Poster: P762 Session: ePoster Date: September 24-26, 2025 Presenter: Vigiser I |
|
Integrin β1 Demarks Circulating Precursors of Brain-residing Antibody-secreting Cells in People with MS
|
Kuiper K, Bogers L, Rip J, et al. |
Oral presentation: O123 Session: Scientific Session 14: Antibody-mediated pathology Date: September 26, 2025 Time: 12:26-12:33 am PDT/03:26-03:33 am EDT Presenter: Smolders J |
|
Early Lymphocyte Reduction as a Potential Marker of Cladribine Efficacy |
Celius E, et al. |
Poster: P2028 Session: ePoster Date: September 24-26, 2025 Presenter: Celius E |
About MAVENCLAD®
MAVENCLAD, approved by the U.S. Food and Drug Administration (FDA) on March 29, 2019, is the first and only short-course oral therapy for the treatment of adults with relapsing-remitting disease (RRMS) and active secondary progressive disease (SPMS). Because of its safety profile, use of MAVENCLAD is generally recommended for patients who have had an inadequate response to, or are unable to tolerate, an alternate drug indicated for the treatment of multiple sclerosis (MS), and MAVENCLAD is not recommended for use in patients with clinically isolated syndrome (CIS). Patients should follow healthcare provider instructions including cancer screening, contraception, and blood tests. The approved dose of MAVENCLAD is 3.5 mg per kg body weight over two years, administered as one treatment course of 1.75 mg per kg per year, each consisting of two treatment weeks. The mechanism by which cladribine exerts its therapeutic effects in patients with multiple sclerosis has not been fully elucidated but is thought to involve cytotoxic effects on B and T lymphocytes through impairment of DNA synthesis, resulting in depletion of lymphocytes. MAVENCLAD causes a dose-dependent reduction in lymphocyte counts followed by recovery.
Because cladribine is cytotoxic, special handling and disposal instructions should be followed.
More than 130,000 patients have been treated with MAVENCLAD since its launch in 2019. MAVENCLAD has been approved in over 80 countries, including the European Union (EU), Canada, Australia and Switzerland, for various relapsing MS indications. Visit www.MAVENCLAD.com for more information.
About Multiple Sclerosis
Multiple sclerosis (MS) is a chronic, inflammatory condition of the central nervous system and is the most common non-traumatic, disabling neurological disease in young adults. It is estimated that approximately 2.9 million people have MS worldwide. While symptoms can vary, the most common symptoms of MS include blurred vision, numbness or tingling in the limbs and problems with strength and coordination. The relapsing forms of MS are the most common.
Merck in Neurology and Immunology
Merck has a long-standing legacy in neurology and immunology, with significant R&D and commercial experience in multiple sclerosis (MS). The company’s current MS portfolio includes two products for the treatment of relapsing MS – Rebif® (interferon beta-1a) and MAVENCLAD® (cladribine tablets). Merck aims to improve the lives of patients by addressing areas of unmet medical needs. In addition to Merck’s commitment to MS, the company also has a pipeline focusing on discovering new therapies that have potential in other neuroinflammatory and immune-mediated diseases, including systemic lupus erythematosus (SLE), cutaneous lupus erythematosus (CLE) and generalized myasthenia gravis (gMG).
About Merck
Merck, a leading science and technology company, operates across life science, healthcare and electronics. More than 62,000 employees work to make a positive difference to millions of people’s lives every day by creating more joyful and sustainable ways to live. From providing products and services that accelerate drug development and manufacturing as well as discovering unique ways to treat the most challenging diseases to enabling the intelligence of devices – the company is everywhere. In 2024, Merck generated sales of € 21.2 billion in 65 countries.
Scientific exploration and responsible entrepreneurship have been key to Merck’s technological and scientific advances. This is how Merck has thrived since its founding in 1668. The founding family remains the majority owner of the publicly listed company. Merck holds the global rights to the Merck name and brand. The only exceptions are the United States and Canada, where the business sectors of Merck operate as MilliporeSigma in life science, EMD Serono in healthcare, and EMD Electronics in electronics.
All Merck press releases are distributed by e-mail at the same time they become available on the Merck website. Please go to www.merckgroup.com/subscribe to register online, change your selection or discontinue this service.
- Duck Creek Technologies 推出端到端 Payments Marketplace 并与 Paymentus 实现全新集成
- Lantronix Announces Percepxion™ Its New Cloud Software Platform for IoT Devices
- 好医生参加“有一种叫云南的机遇”招商推介会
- eXp Realty Launches in Peru, Creating Unmatched Opportunities for Agents to Build Their Future
- 勇闯雪山极限挑战,老板燃气灶以安全大火力树立“极地品质”标杆
- 重磅!新汇制药猴头健胃灵片治疗CNAG取得诊疗新进展
- OPPET:全球能源数字化革命正式启幕
- 新疆威兹曼双C动感丰胸 从手术到康复的全周期关怀
- 学术与资本双轮驱动,FireFly-隐私计算赛道的资本宠儿
- DauggeKemmi携pH6.5科学洗护闪耀它博会,引领宠物健康养护新风潮
- A ‘Complete Revolution’ in Manufacturing: NTT DATA Research Reveals GenAI’s Transformative Potential
- 平安养老险新疆分公司:开展系列“3.15”消费者权益保护宣传日活动
- 京东限时大额补贴 木林森照明立式护眼灯成 618 健康护眼利器
- 冲上热搜!太“上头”!除了这碗粉,它还坐拥“全球第一”!
- 经济学家朱民谈比亚迪落泪:中国制造业崛起是一代人的骄傲
- 江西省数字能源联合会成立大会在南昌举行
- 爱可声助听器在欧美国家广受好评
- DTC2024 TCL华星发布先进显示技术品牌APEX,量产印刷OLED专显屏
- 模拟光源控制器模拟恒流光源控制器恒流源电源控制调节盒
- 大女主爽剧《主妇的觉醒》杀青,女性觉醒互助引热议
- 新专《王Love, Lord》惊喜来袭 林宥嘉鼓励歌迷“以爱为王”
- 九科信息在国央企RPA市场份额排名第一,四大RPA行业皆排名前三
- 张家港市赛博科技有限公司——引领全球创新解决方案
- Mavenir为印度Vodafone Idea大规模部署Open RAN进行商业阶段准备
- 24小时主动守护!A.O.史密斯智慧「瀞」厨房,让安全隐患无处可藏
- 中医药现代化服务的普惠探索,御君方的智慧服务与家庭健康管理的融合之道
- 中国开源软件推进联盟携手IBM发布《可信赖的企业级生成式AI白皮书》
- 双奥书法家创作420福字拼图“滑马”亮相北京,为2025滑县马拉松注入传统文化内涵
- 一张沙发的价值革命:解读顾家家居如何用“全皮沙发”打破行业困境
- Capsa Healthcare Announces New Interface to EMIS ProScript Connect for RoboWall Prescription Pickup
推荐
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
-
 透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
-
 王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
-
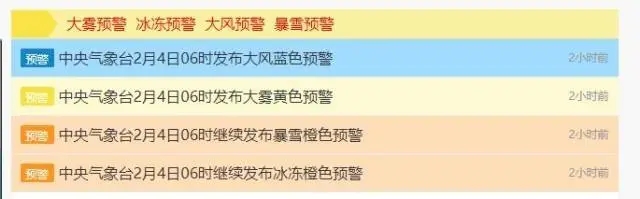 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
-
 新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
-
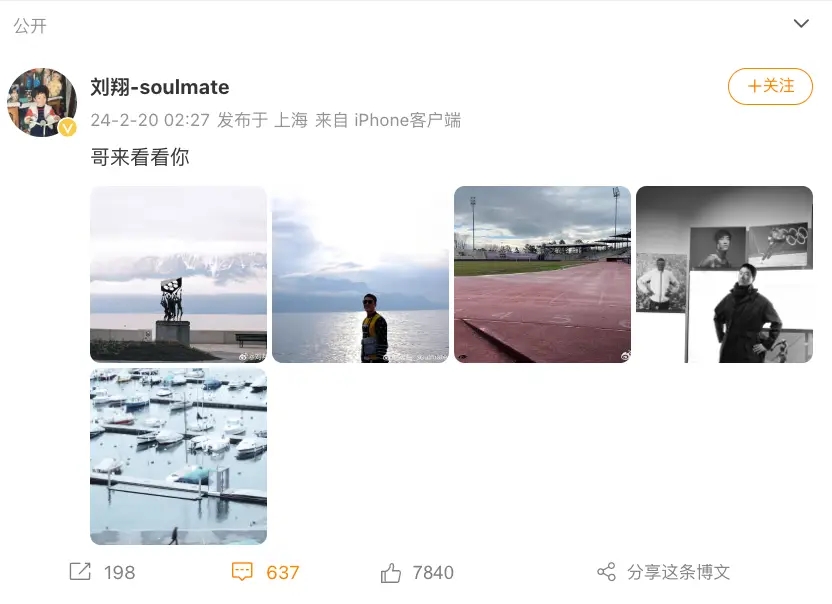 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯

