Teva Announces FDA Approval and Launch of Generic Saxenda® (liraglutide injection) – First Generic G

- Generic Saxenda® is the first-ever generic GLP-1 indicated for weight loss, addressing increased demand for this category of therapies in the U.S. market.
- This approval and launch add to Teva’s continued commitment to its complex generic medicine portfolio as part of its Pivot to Growth Strategy.
- Liraglutide injection is indicated for adults with obesity or overweight (excess weight) who also have weight related medical problems, and pediatric patients (12-17 years) with a weight greater than 60 kg and obesity to help them lose weight and keep the weight off.
PARSIPPANY, N.J., Aug. 28, 2025 (GLOBE NEWSWIRE) -- Teva Pharmaceuticals, Inc., a U.S. affiliate of Teva Pharmaceutical Industries Ltd. (NYSE and TASE: TEVA), announced today the FDA approval and U.S. launch of a generic version of Saxenda®1 (liraglutide injection).
“With this approval, and by launching a generic for Saxenda® (liraglutide injection), we will provide patients in the U.S. the first ever generic GLP-1 product specifically indicated for weight loss,” said Ernie Richardsen, SVP, Head of U.S. Commercial Generics at Teva. “This is the fifth first-to-market entry of a Teva generic this year and is an important addition to Teva's diverse complex generics portfolio, demonstrating once again our proven ability to sustain a world class Generics Powerhouse.”
Saxenda® had annual sales of $165 million as of June 2025.2
What is Liraglutide Injection?
Liraglutide Injection is a glucagon like peptide 1 (GLP-1) receptor agonist indicated in combination with a reduced calorie diet and increased physical activity to reduce excess body weight and maintain weight reduction long term in:
- Adults and pediatric patients aged 12 years and older with body weight greater than 60 kg and obesity.
- Adults with overweight in the presence of at least one weight-related comorbid condition.
- Liraglutide injection should be used with a reduced calorie diet and increased physical activity.
- Liraglutide injection is not recommended for people who also take liraglutide or other medicines called glucagon-like peptide-1 (GLP-1) receptor agonists.
- It is not known if liraglutide injection is safe and effective in children under 12 years of age.
- It is not known if liraglutide injection is safe and effective in children aged 12 to 17 years with type 2 diabetes.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about liraglutide injection?
Serious side effects may happen in people who take liraglutide injection, including:
Possible thyroid tumors, including cancer. Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer. In studies with rats and mice, liraglutide injection and medicines that work like liraglutide injection caused thyroid tumors, including thyroid cancer. It is not known if liraglutide injection will cause thyroid tumors or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people.
Do not use liraglutide injection if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC), or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Who should not take liraglutide injection?
Do not receive liraglutide injection if:
- you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- you have had a serious allergic reaction to liraglutide or any of the ingredients in liraglutide injection. See “What are the possible side effects of liraglutide injection?” for symptoms of a serious allergic reaction.
Before taking liraglutide injection tell your healthcare provider about all of your medical conditions, including if you:
- have or have had problems with your pancreas
- have severe problems with your stomach, such as slowed emptying of your stomach (gastroparesis) or problems with digesting food.
- are scheduled to have surgery or other procedures that use anesthesia or deep sleepiness (deep sedation).
- have or have had depression or suicidal thoughts, or mental health issues.
- are breastfeeding or plan to breastfeed. It is not known if liraglutide passes into your breast milk. You and your healthcare provider should decide if you will use liraglutide injection or breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Liraglutide injection may affect the way some medicines work and some other medicines may affect the way liraglutide injection works.
Tell your healthcare provider if you take diabetes medicines, especially insulin and sulfonylurea medicines. Talk with your healthcare provider if you are not sure if you take any of these medicines. Know the medicines you take. Keep a list to show your healthcare provider and pharmacist when you get a new medicine.
What are the possible side effects of liraglutide injection?
Liraglutide Injection may cause serious side effects, including:
- inflammation of the pancreas (pancreatitis). Stop using liraglutide injection and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your stomach area (abdomen) to your back
- increased risk of low blood sugar (hypoglycemia) in adults with type 2 diabetes especially those who also take medicines to treat type 2 diabetes mellitus such as an insulin or a sulfonylureas and in children who are 12 years of age and older without type 2 diabetes mellitus. Low blood sugar in patients with adults with type 2 diabetes and in children without type 2 diabetes mellitus who receive liraglutide injection can be both a serious and common side effect. Talk to your healthcare provider about how to recognize and treat low blood sugar. You should check your blood sugar before you start taking liraglutide injection and while you take liraglutide injection.
- Signs and symptoms of low blood sugar may include:
- dizziness or light-headedness
- sweating
- confusion or drowsiness
- headache
- blurred vision
- slurred speech
- shakiness
- fast heartbeat
- anxiety, irritability, or mood changes
- hunger
- weakness
- feeling jittery
Talk to your healthcare provider about how to recognize and treat low blood sugar. You should check your blood sugar before you start taking liraglutide injection and while you take liraglutide injection.
- increased heart rate. Liraglutide injection can increase your heart rate while you are at rest. Your healthcare provider should check your heart rate while you take liraglutide injection. Tell your healthcare provider if you feel your heart racing or pounding in your chest and it lasts for several minutes.
- dehydration leading to kidney problems. Diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration) which may cause kidney problems. It is important for you to drink fluids to help reduce your chance of dehydration. Tell your healthcare provider right away if you have nausea, vomiting, or diarrhea that does not go away
- severe stomach problems. Stomach problems, sometimes severe, have been reported in people who use liraglutide injection. Tell your healthcare provider if you have stomach problems that are severe or will not go away.
- serious allergic reactions. Stop using liraglutide injection, and get medical help right away if you have any symptoms of a serious allergic reaction including:
- swelling of your face, lips, tongue, or throat
- problems breathing or swallowing
- severe rash or itching
- fainting or feeling dizzy
- very rapid heartbeat
- gallbladder problems. Liraglutide injection may cause gallbladder problems including gallstones. Some gallbladder problems need surgery. Call your healthcare provider if you have any of the following symptoms:
- pain in your upper stomach (abdomen)
- fever
- yellowing of your skin or eyes (jaundice)
- clay-colored stools
- depression or thoughts of suicide. You should pay attention to any mental changes, especially sudden changes, in your mood, behaviors, thoughts, or feelings. Call your healthcare provider right away if you have any mental changes that are new, worse, or worry you.
- food or liquid getting into the lungs during surgery or other procedures that use anesthesia or deep sleepiness (deep sedation). Liraglutide injection may increase the chance of food getting into your lungs during surgery or other procedures. Tell all your healthcare providers that you are taking liraglutide injection before you are scheduled to have surgery or other procedures.
The most common side effects of liraglutide injection in adults include:
- nausea
- diarrhea
- constipation
- vomiting
- injection site reaction
- low blood sugar (hypoglycemia)
- headache
- upset stomach (dyspepsia)
- tiredness (fatigue)
- dizziness
- stomach pain
- change in enzyme (lipase) levels in your blood
Additional common side effects in children are fever and gastroenteritis.
Talk to your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of liraglutide injection. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Please read the Medication Guide in the full Prescribing Information, including Boxed Warning.
About Teva
Teva Pharmaceutical Industries Ltd. (NYSE and TASE: TEVA) is a leading innovative biopharmaceutical company, enabled by a world-class generics business. For over 120 years, Teva’s commitment has never wavered. From innovating in the fields of neuroscience and immunology to providing complex generic medicines, biosimilars and pharmacy brands worldwide, Teva is dedicated to addressing patients’ needs, now and in the future. At Teva, We Are All In For Better Health. To learn more about how, visit www.tevapharm.com.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, which are based on management’s current beliefs and expectations and are subject to substantial risks and uncertainties, both known and unknown, that could cause our future results, performance or achievements to differ significantly from that expressed or implied by such forward-looking statements. You can identify these forward-looking statements by the use of words such as “should,” “expect,” “anticipate,” “estimate,” “target,” “may,” “project,” “guidance,” “intend,” “plan,” “believe” and other words and terms of similar meaning and expression in connection with any discussion of future operating or financial performance. Important factors that could cause or contribute to such differences include risks relating to: the development, launch and commercial success of our generic version to Saxenda® (liraglutide injection); our ability to successfully compete in the marketplace, including that we are substantially dependent on our generic products; concentration of our customer base and commercial alliances among our customers; competition faced by our generic medicines from other pharmaceutical companies and changes in regulatory policy that may result in additional costs and delays; delays in launches of new generic products; our ability to develop and commercialize additional pharmaceutical products; our ability to successfully execute our Pivot to Growth strategy, including to expand our innovative and biosimilar medicines pipeline and profitably commercialize the innovative medicines and biosimilar portfolio, whether organically or through business development; and other factors discussed in this press release, in our Quarterly Report on Form 10-Q for the second quarter of 2025 and in our Annual Report on Form 10-K for the year ended December 31, 2024, including in the section captioned “Risk Factors.” Forward-looking statements speak only as of the date on which they are made, and we assume no obligation to update or revise any forward-looking statements or other information contained herein, whether as a result of new information, future events or otherwise. You are cautioned not to put undue reliance on these forward-looking statements.
Teva Media Inquiries:
TevaCommunicationsNorthAmerica@tevapharm.com
Teva Investor Relations Inquiries:
TevaIR@tevapharm.com
1 Saxenda® is a registered trademark of Novo Nordisk.
2 According to IQVIA data
- 望京之星|时代商务钜制 以远见缔造未来
- “共享创新 共赢未来”杭甬双城产融合作大会暨高层次人才项目路演圆满完成
- 利德治疗仪 春季腰椎间盘突出康复的智慧之选
- 亚裔定制盼生派C9NMN引爆市场,预售千瓶瞬时被抢空!
- 望繁信科技携流程智能解决方案亮相CNDS 2024新能源产业数智峰会
- CLORIS凯伦诗按摩椅震撼上市,重新定义家庭按摩享受
- 推进“以旧换新”政策落地,远程加速实现“0元油换电”
- 海正药业携喜美欣入列京东健康首批科学护肝联盟品牌
- LambdaTest和QualityKiosk Technologies携手提升企业质量保证水平
- 东莞市恒锋塑胶制品有限公司——全品类亚克力产品专业生产及研发
- 衡泰信GOLFJOY:打破场地局限,畅享高尔夫非凡乐趣!
- 馨悦采耳养生保健馆:舒适环境,专业服务,打造全新养生体验!
- 叶耀玲新中式茶饮产品获“助农杯”创新成果奖,并获安溪县委吴毓舟书记高度肯定
- PUMA入选Laureus体育公益指数
- 新质生产力 安保医疗连获6项创新类大奖!
- 邓超予携《我道人间好》参与北京卫视影视频道春晚录制,诠释非遗文化之美
- 东方菁英云校:给孩子一条升学捷径
- 跨越山海·聚合设计力量|2024金牌整家明星设计师大赛圆满收官
- 扎耶德可持续发展奖论坛首次在中国成功举办
- 年货进京1号线 戈壁红驼从草原到首都,共赴乡村振兴新征程——热烈庆祝内蒙古戈壁红驼生物科技有限责任公司入选北京地铁1号线年货进京专列
- 以工商数据司法信息知识产权等数百种维度构建的企业数据库在商业上的应用
- 中华老字号的“不老”传奇 ——白山方大集团品牌诚信建设纪实
- 国家安全教育进校园-石家庄铁路公安处衡水站派出所联合开展主题宣传活动
- 第七届“绽放杯”5G应用征集大赛5G+智慧城市专题赛圆满收官
- 新质生产力探索| AICG浪潮下的3D打印与3D扫描技术
- 海能达将携“公专融合”全系创新产品亮相巴展MWC
- 药易购强化非药大健康布局 设立5000万全资子公司金豆豆构建产业生态闭环
- Nyxoah Announces Proposed Offering of Ordinary Shares
- European High Growth Opportunities Securitization Fund评论Tony G Co-Investment Holdings Ltd.发布的新闻稿
- 鸿誉出国:让世界触手可及!
推荐
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
-
 新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
-
 透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
-
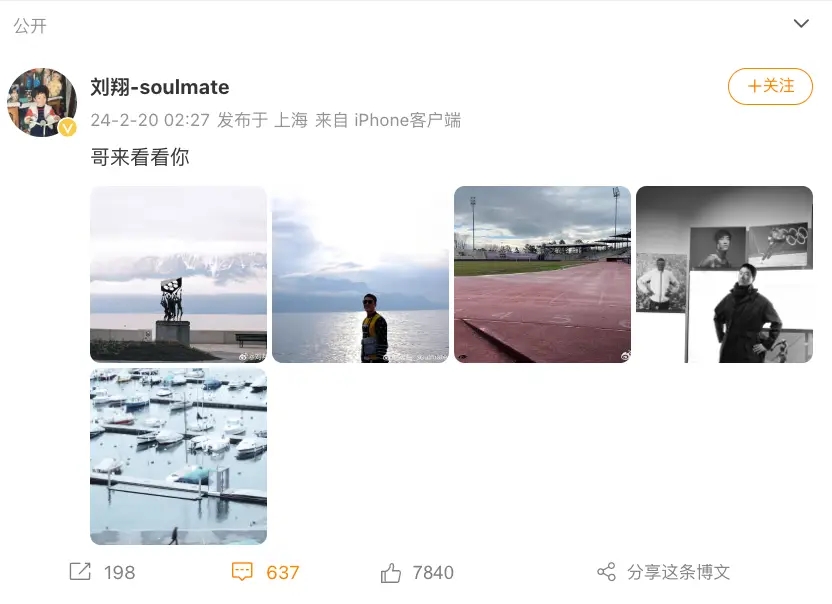 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
-
 王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
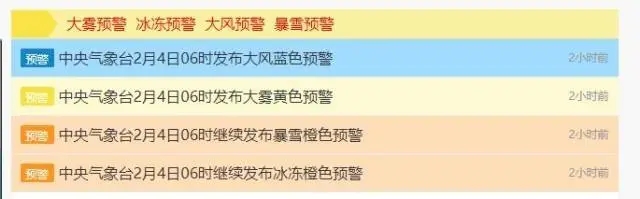 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯

