Zymeworks Announces NMPA Approval of Zanidatamab in China for Adults
Zymeworks Announces NMPA Approval of Zanidatamab in China for Adults with Previously Treated, Unresectable or Metastatic HER2-high expression (IHC3+) Biliary Tract Cancer
- Zanidatamab is the first and only dual HER2-targeted bispecific antibody approved for HER2+ biliary tract cancer in China; conditional approval based on the results of the HERIZON-BTC-01 clinical study
- $20 million milestone payment to be received from BeOne Medicines; Zymeworks remains eligible to receive up to $144 million in additional development and commercial milestones
VANCOUVER, British Columbia, May 30, 2025 (GLOBE NEWSWIRE) -- Zymeworks Inc. (Nasdaq: ZYME), a clinical-stage biotechnology company developing a diverse pipeline of novel, multifunctional biotherapeutics to improve the standard of care for difficult-to-treat diseases, including cancer, inflammation, and autoimmune disease, today announced that the National Medical Products Administration (NMPA) in China has approved zanidatamab for the treatment of patients with previously treated, unresectable or metastatic HER2-positive (HER2+) biliary tract cancer (BTC). The conditional approval marks the first and only1 dual HER2-targeted bispecific antibody approved for HER2-high expression (IHC3+) BTC in China. Zymeworks' collaboration partner, BeOne Medicines Ltd. (formerly BeiGene, Ltd), obtained the conditional approval under the terms of its Asia Pacific license and collaboration agreement with Zymeworks. Continued approval of this indication will depend on the verification of clinical benefit in the patient population through ongoing confirmatory trials.
“Zanidatamab's conditional approval in China is a meaningful advancement for patients living with HER2-positive BTC, a population with historically high unmet need and poor prognoses,” said Kenneth Galbraith, Chair and Chief Executive Officer of Zymeworks. “This milestone affirms the strength of zanidatamab's clinical potential and reflects our continued focus on translating innovation into real impact for patients around the globe. We are deeply grateful to our partners at BeOne Medicines, and to the patients, families, and clinical teams whose contributions have made this milestone a reality. As Zymeworks continues to advance our broader development programs and R&D pipeline, we remain committed to realizing zanidatamab's potential to transform the standard of care across HER2-expressing cancers.”
Under the terms of its agreement with BeOne Medicines, Zymeworks has received $61 million in upfront and milestone payments, as well as certain co-development funding for zanidatamab clinical studies. Zymeworks is entitled to receive a $20 million milestone payment in connection with the NMPA approval of zanidatamab, and is eligible to receive up to $144 million in additional development and commercial milestones. Zymeworks is also eligible to receive tiered royalties of up to 19.5% of net sales in BeOne Medicine's territories.
Zanidatamab was approved by the U.S. Food and Drug Administration (FDA) in November 2024 for the treatment of adults with previously treated, unresectable or metastatic HER2+ (IHC 3+) BTC. In April 2025, Zymeworks' partner, Jazz Pharmaceuticals, announced that the European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion recommending the approval of zanidatamab for the treatment of advanced HER2+ BTC.
About Biliary Tract Cancer
Biliary tract cancers (BTC), including gallbladder cancer and intrahepatic and extrahepatic cholangiocarcinoma, account for approximately 3% of all digestive system tumors and are often associated with a poor prognosis2,3,4. Approximately 11%-25.2% of patients with BTC are HER2-positive5,6,7. The human epidermal growth factor receptor 2 (HER2) is a well-validated target for antitumor therapy in other cancers3,8. The incidence rate of BTC is on the rise globally, in particular in Asian countries and regions.9
About zanidatamab
Zanidatamab is a dual HER2-targeted bispecific antibody that simultaneously binds extracellular domains 2 and 4 on separate HER2 monomers (binding in trans). Binding of zanidatamab with HER2 results in internalization leading to a reduction of the receptor on the cell surface. Zanidatamab induces complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP). These mechanisms result in tumor growth inhibition and tumor cell death.10
About Zymeworks Inc.
Zymeworks is a global clinical-stage biotechnology company committed to the discovery, development, and commercialization of novel, multifunctional biotherapeutics. Zymeworks' mission is to make a meaningful difference in the lives of people impacted by difficult-to-treat conditions such as cancer, inflammation, and autoimmune disease. The Company's complementary therapeutic platforms and fully integrated drug development engine provide the flexibility and compatibility to precisely engineer and develop highly differentiated antibody-based therapeutic candidates. Zymeworks engineered and developed zanidatamab, a HER2-targeted bispecific antibody using the Company's proprietary Azymetric™ technology. Zymeworks has entered into separate agreements with BeOne Medicines Ltd. (formerly BeiGene, Ltd.) and Jazz Pharmaceuticals Ireland Limited, granting each exclusive rights to develop and commercialize zanidatamab in different territories. The U.S. FDA granted accelerated approval and China's NMPA granted conditional approval for zanidatamab to treat adults with previously-treated, unresectable or metastatic HER2-positive (IHC 3+) biliary tract cancer. Zanidatamab is the first and only dual HER2-targeted bispecific antibody approved for HER2-positive BTC in the U.S. and China. Zanidatamab is currently under regulatory review in the EU for second-line BTC and is being evaluated in multiple global clinical trials as a potential best-in-class treatment for patients with multiple HER2-expressing cancers. Zymeworks is rapidly advancing a robust pipeline of wholly-owned product candidates, leveraging its expertise in both antibody-drug conjugates and multispecific antibody therapeutics targeting novel pathways in areas of significant unmet medical need. Phase 1 studies for ZW171 and ZW191 are now actively recruiting with an investigational new drug application for ZW251 planned for mid-2025. In addition to Zymeworks' pipeline, its therapeutic platforms have been further leveraged through strategic partnerships with global biopharmaceutical companies. For information about Zymeworks, visit www.zymeworks.com and follow @ZymeworksInc on X.
Cautionary Note Regarding Forward-Looking Statements
This press release includes “forward-looking statements” or information within the meaning of the applicable securities legislation, including Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements in this press release include, but are not limited to, statements that relate to the efficacy and safety of zanidatamab; ongoing clinical studies and regulatory reviews; the anticipated benefits of the collaboration agreement with BeOne Medicines, including Zymeworks' ability to receive any future milestone payments and royalties thereunder; the potential addressable market of zanidatamab; the timing of and results of interactions with regulators; Zymeworks' clinical development of its product candidates and enrollment in its clinical trials; the timing and status of ongoing and future studies and the related data; expectations regarding future regulatory filings and approvals and the timing thereof; potential safety profile and therapeutic effects of zanidatamab; the commercial potential of technology platforms and product candidates; Zymeworks' ability to satisfy potential regulatory and commercial milestones with existing and future partners and anticipated continued receipt of revenue from existing and future partners. When used herein, words such as “plan”, “believe”, “expect”, “may”, “anticipate”, “potential”, “will”, “continues”, and similar expressions are intended to identify forward-looking statements. In addition, any statements or information that refer to expectations, beliefs, plans, projections, objectives, performance or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking. All forward-looking statements are based upon Zymeworks' current expectations and various assumptions. Zymeworks believes there is a reasonable basis for its expectations and beliefs, but they are inherently uncertain. Zymeworks may not realize its expectations, and its beliefs may not prove correct. Actual results could differ materially from those described or implied by such forward-looking statements as a result of various factors, including, without limitation: clinical trials, including any required confirmatory trials, may not demonstrate safety and efficacy of any of Zymeworks' or its collaborators' product candidates; any of Zymeworks' or its partners' product candidates may fail in development, may not receive required regulatory approvals, or may be delayed to a point where they are not commercially viable; conditional regulatory approval may be withdrawn or revoked if any of Zymeworks' or its partners' product candidates fail to satisfy the requirements of any such conditional regulatory approvals; regulatory agencies may impose additional requirements or delay the initiation of clinical trials; the impact of new or changing laws and regulations; market conditions, including the impact of tariffs; potential negative impacts of FDA regulatory delays and uncertainty and new policies implemented under the current administration, including executive orders, changes in the leadership of federal agencies such as the FDA, staff layoffs, budget cuts to agency programs and research, and changes in drug pricing controls; the impact of pandemics and other health crises on Zymeworks' business, research and clinical development plans and timelines and results of operations, including impact on its clinical trial sites, collaborators, and contractors who act for or on Zymeworks' behalf; clinical trials and any future clinical trials may not demonstrate safety and efficacy of any of Zymeworks' or its collaborators' product candidates; inability to maintain or enter into new partnerships or strategic collaborations; and the factors described under “Risk Factors” in Zymeworks' quarterly and annual reports filed with the Securities and Exchange Commission (copies of which may be obtained at www.sec.gov and www.sedarplus.ca).
Although Zymeworks believes that such forward-looking statements are reasonable, there can be no assurance they will prove to be correct. Investors should not place undue reliance on forward-looking statements. The above assumptions, risks and uncertainties are not exhaustive. Forward-looking statements are made as of the date hereof and, except as may be required by law, Zymeworks undertakes no obligation to update, republish, or revise any forward-looking statements to reflect new information, future events or circumstances, or to reflect the occurrences of unanticipated events.
Investor inquiries:
Shrinal Inamdar
Senior Director, Investor Relations
(604) 678-1388
ir@zymeworks.com
Media inquiries:
Diana Papove
Senior Director, Corporate Communications
(604) 678-1388
media@zymeworks.com
_________________________________
1 According to publicly available information, as of the approval announcement on May 29, 2025, zanidatamab is the only HER2-targeting bispecific antibody approved by the National Medical Products Administration (NMPA) for HER2-high-expression biliary tract cancer (BTC).
2 Chinese Society of Clinical Oncology (CSCO). Diagnosis and Treatment Guidelines for Biliary Malignant Tumors (2024).
3 Vogel A, Bridgewater J, Edeline J, et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment, and follow-up. Ann Oncol. 2023;34(2):127-40. doi:10.1016/j.annonc.2022.10.506.
4 Chakrabarti, S., Kamgar.doi.org/10.3390/cancers12082039.
5 Choong-kun Lee, et al. 2025 ASCO GI Abstr. #629.
6 Hiraoka N, et al. Hum Pathol. 2020 Nov:105:9-19.
7 Vivaldi C, et al. Oncologist. 2020 Oct;25(10):886-893.
8 Meric-Bernstam, F., Beeram, et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study. The Lancet Oncology. 2022; doi: 10.1016/S1470-2045(22)00621-0.
9 Chinese Society of Clinical Oncology (CSCO) Biliary Tumor Expert Committee Expert consensus on precise detection and molecular diagnosis of biliary malignant tumors [J]. Journal of Clinical Oncology, 2024, 29 (8): 797-804.
10 Weisser NE, Sanches M, Escobar-Cabrera E et al. An anti-HER2 biparatopic antibody that induces unique HER2 clustering and complement-dependent cytotoxicity. Nature Communications. 2023 Mar 13;14(1):1394. doi: 10.1038/s41467-023-37029-3.
- 帝国大厦举办一年一度的爬楼赛,由Starbucks赞助并由Challenged Athletes Foundation提供支持
- 力与美之巅峰对决“政彤健身杯”DMS 健美比赛许昌站震撼收官!
- 官宣|贵族水正式面世,开启饮用水时代的迭代升级
- 吴尊萨巴伦卡法网合影 原来是帮儿子MAX追星
- 俄运输机坠毁,74人全部遇难!俄罗斯称是被乌克兰导弹击落
- 嘀嗒来电移动充电服务 99元/5度电
- Spatial发布 Data Prep
- WS外贸新生:WhatsApp工具,一个小白的外贸蜕变之旅
- 门业十大品牌群邦门业 百万家庭的信任之选
- Kao Corporation披露《董事会对股东提案的意见及Kao提升企业价值的策略》
- 招商云墨 | 高新CID曈北最后价值高地,几人将收藏?
- 全国首次,历史性突破!今天,他们在珠港澳御风而行!
- 用音乐传递美好,胡桃里十周年“艺术演出季”点亮城市夜生活
- 老板电器发布首个烹饪大模型“食神”,再次引领烹饪变革
- Mastercard and FreedomPay Unlock Global Commerce With New Payment Gateway Partnership
- Ella陈嘉桦推出全新单曲〈南方的月亮〉,1/22在各大数位音乐平台全网上线
- Kelly陈慧琳全新国语新歌《谢谢你陪我那么久》 演唱会上海站首唱
- 恒昌医药:第五届“江琎奖学金”揭晓!科研创业双星闪耀
- 梦中情车!张柏芝力荐极氪009 直言“满足了我的梦想”
- 第五届《中华罗氏春晚》新闻公告
- 《金雕飞起的乌兰察布》——大型沉浸式情景歌舞剧的制作背后
- PayMate Announces Intent to Acquire DigiAsia
- 中国人寿财险怀化市中心支公司开展 “3.15”总经理接访活动
- 中国国际救援中心集团董事局康海秀主席率团再次访问柬埔寨王国驻华总领事馆
- 营运中心“星光闪耀时”——第二季度评优盛典暨沙盘团建精彩瞬间
- 中国黑莓采摘节——中国自主研发的第一黑莓品种
- 智领未来,共赴新章 丰尚智慧园项目开工仪式顺利举行
- OSL Appointed as the First Exchange and Sub-Custodian Partner for China AMC’s Inaugural Spot BTC/ETH
- 七大跨境收款平台对比,出海卖家应该怎么选?
- 中国电信发布全自研视频生成大模型 完成全模态体系构建,首届TeleAI 开发者大会在穗举办
推荐
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
 抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
-
 透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
-
 周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
-
 王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
-
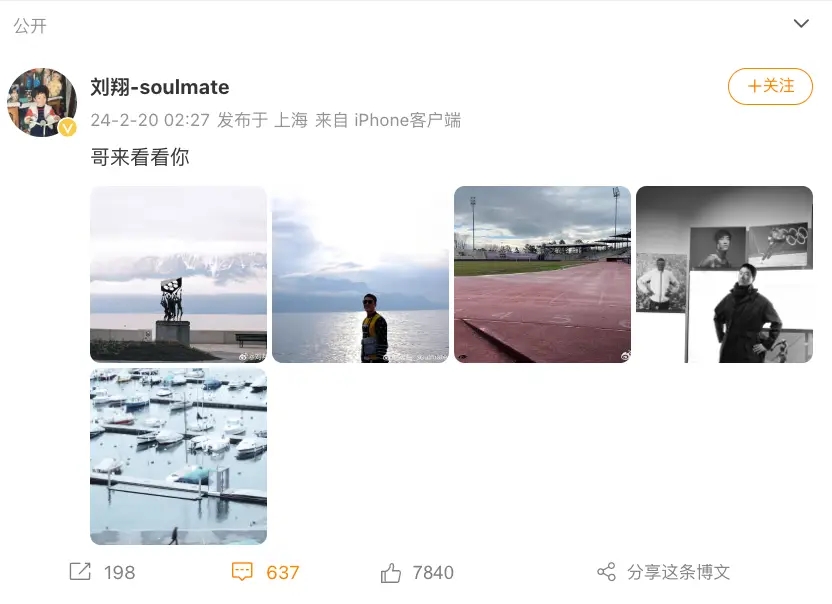 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯

