Sabin Begins Marburg Vaccine Trial in U.S.
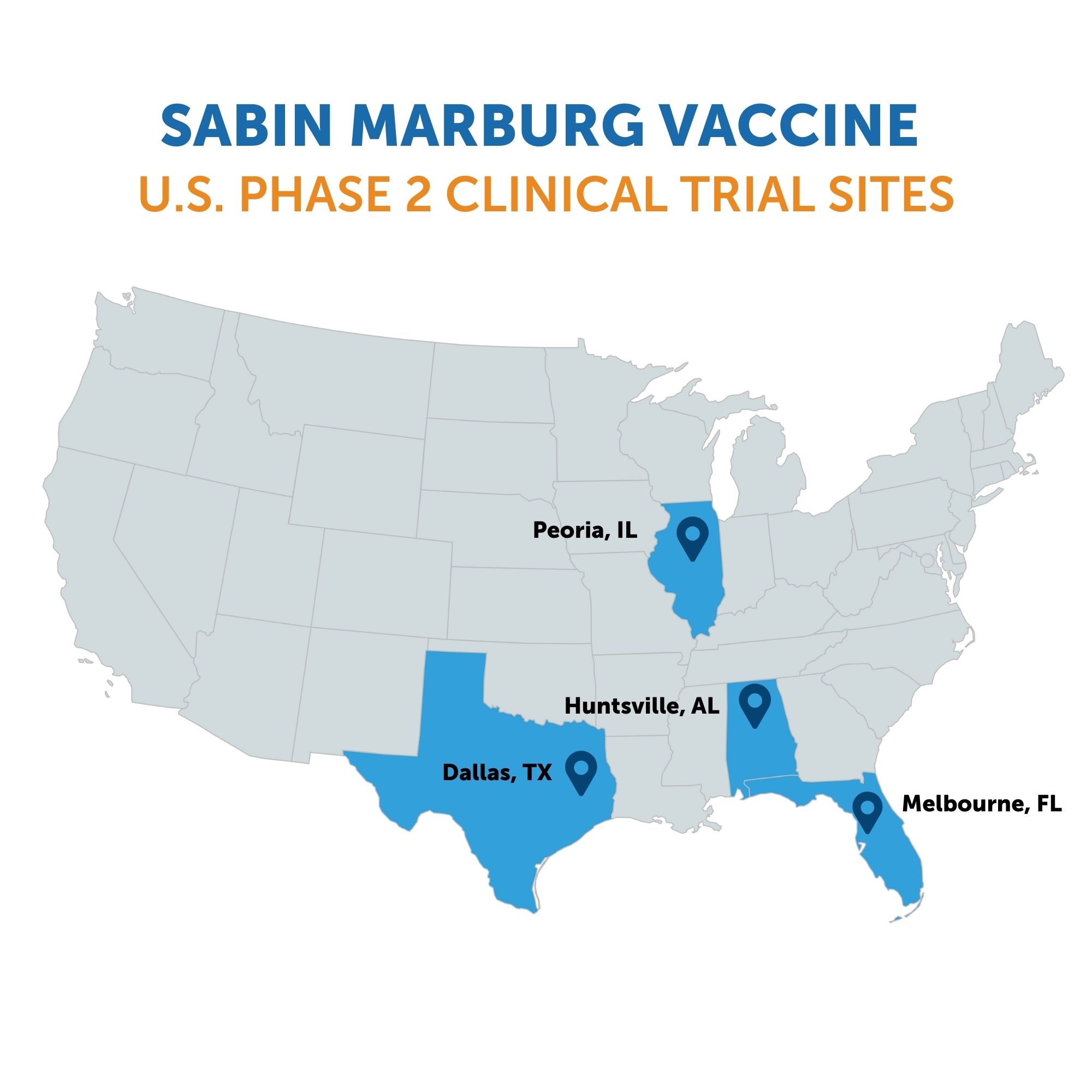
WASHINGTON, April 16, 2025 (GLOBE NEWSWIRE) -- The Sabin Vaccine Institute has launched a multi-site Phase 2 clinical trial in the U.S. for its Marburg vaccine candidate, administering doses to the first participants in Melbourne, Florida. This trial builds on ongoing Phase 2 testing in Kenya and Uganda, with initial findings from that research expected in the coming months.
Sabin's vaccine development efforts, including clinical trials, are becoming increasingly critical as Marburg outbreaks grow in frequency, underscoring the urgent need for vaccines to protect those at highest risk. Sabin supported an open-label Phase 2 clinical trial sponsored by the Rwanda Biomedical Centre (RBC) by supplying the investigational vaccine during Rwanda's 2024 Marburg outbreak. More than 1,700 individuals — primarily frontline health care workers — were vaccinated, with first doses arriving within nine days of the outbreak being declared. Data from the RBC trial will be shared with Sabin to support the vaccine's licensure.
Rwanda's outbreak ended on December 20 with a case fatality rate of 23%, lower than the historical average of 50%. Fatality rates in outbreaks can vary due to multiple factors, including greater surveillance, prompt detection, supportive care, and the overall response effort. On January 20, Tanzania declared an outbreak of Marburg virus disease, which ended on March 13.
Currently, there are no approved vaccines for Marburg virus disease.
For the U.S. clinical trial that began this week, Sabin will recruit 200 volunteers aged 18 to 70 across four locations – in addition to Melbourne, the vaccine will be tested at sites in Dallas, Texas; Huntsville, Alabama; and Peoria, Illinois. The randomized, placebo-controlled, double-blind trial will continue to evaluate safety and immunogenicity, and will monitor vaccinated volunteers for a year.
“Recent outbreaks highlight the urgent need to strengthen our defenses against this deadly and unforgiving disease,” says Amy Finan, Sabin's Chief Executive Officer. “Sabin's Phase 2 clinical trials will generate essential data to move this vaccine closer to licensure — and offer a potentially life-saving tool where none exists.”
Marburg is a filovirus, in the same family as the virus that causes Ebola. Like Ebola, Marburg virus disease spreads via direct contact with the blood or other bodily fluids of infected individuals. It is highly virulent and causes hemorrhagic fever.
“Conducting clinical trials in Africa is key to evaluating the vaccine in regions where Marburg and other filoviruses are most common or endemic," notes Kelly Warfield, Sabin's President of Research & Development. "The U.S. trial will give us vital safety and immune response data for non-endemic populations, helping us better prepare for outbreaks and spread of this disease.”
Based on the cAd3 platform, Sabin's single-dose investigational Marburg vaccine was found to be promising in Phase 1 clinical and non-clinical studies, with results showing it to be safe, while eliciting rapid and robust immune responses.
The Marburg vaccine trials are supported by the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services, under multi-year contracts between the organizations.
BARDA and Sabin began working together in September 2019 to develop monovalent vaccine candidates for Marburg virus and Sudan virus diseases. To date, Sabin has received around $252 million in contract awards from BARDA for furthering vaccine research and development against these two disease threats.
A Phase 2 clinical trial for Sabin's Sudan virus vaccine is underway in Uganda and Kenya. The cAd3 Sudan vaccine candidate will also be tested among adult volunteers in the U.S. later this year.
This project has been supported in whole or in part with federal funds from the Department of Health and Human Services; Administration for Strategic Preparedness and Response; Center for the Biomedical Advanced Research and Development Authority (BARDA), under contract numbers 75A50119C00055 and 75A50123C00010.
For information about Sabin's Phase 2 Marburg vaccine trials, visit:
Clinicaltrials.gov -- NCT06620003 (U.S. trial)
Clinical.trials.gov -- NCT05817422 (Uganda and Kenya trial)
Pan African Clinical Trials Registry -- PACTR202306534727467 (Uganda and Kenya trial)
About the Sabin Vaccine Institute
The Sabin Vaccine Institute is a leading advocate for expanding vaccine access and uptake globally, advancing vaccine research and development, and amplifying vaccine knowledge and innovation. Unlocking the potential of vaccines through partnership, Sabin has built a robust ecosystem of funders, innovators, implementers, practitioners, policy makers and public stakeholders to advance its vision of a future free from preventable diseases. As a non-profit with three decades of experience, Sabin is committed to finding solutions that last and extending the full benefits of vaccines to all people, regardless of who they are or where they live. At Sabin, we believe in the power of vaccines to change the world. For more information, visit www.sabin.org and follow us on X @SabinVaccine.
About Sabin's Vaccine R&D and the cAd3 Platform
In August 2019, Sabin announced exclusive agreements with GSK for Sabin to advance the development of the prophylactic candidate vaccines against the deadly Zaire ebolavirus, Sudan virus, and Marburg virus. The three candidate vaccines were initially developed collaboratively by the U.S. National Institutes of Health and Okairos, which was acquired by GSK in 2013. The candidate vaccines, based on GSK's proprietary cAd3 (Chimpanzee Adenovirus Type 3) platform, were further developed by GSK, including the Phase 2 development for the Zaire ebolavirus vaccine. Under the agreements between GSK and Sabin, Sabin exclusively licensed the technology for all three candidate vaccines and acquired certain patent rights specific to these vaccines.
Media Contact:
Monika Guttman
Senior Media Relations Specialist
Sabin Vaccine Institute
+1 (202) 621-1691
press@sabin.org
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/cdb8a6cd-2ff2-49bd-aeb4-43e859c1b44f
- 启航2025——重磅推荐新六法论创始人陈其旋
- Instagram精准引流工具,ins全球粉丝采集软件,ig私信助手/ins协议号
- Visa发行第100亿枚代币,为全球电子商务带来400亿美元增量收入
- 新时代机遇下,成都高新区如何展开IVD产业生态圈的全新构想?
- 海亮科技亮相第84届中国教装展 尽显“生于校园 长于校园”教育基因
- 专业驱动,科学护肤——绽妍生物携绽小妍亮相CDA大会
- Andersen Global通过新增成员公司加强在亚洲的纽带关系
- 全女性社交图鉴,好时光即将开启新旅程
- Textron Aviation的Cessna Skycourier被Hinterland Aviation选中用于扩充机队,为澳大利亚的区域连接带来革命性变化
- 凝聚行业共识,共启新型电力产业高质量发展新征程
- 凤凰网河北报道:技术破局+京冀协同:卓翼智能撬动低空蓝海
- AMD、博通、思科、Google、惠普、英特尔、Meta和微软成立超级加速器链(Ultra Accelerator Link,UALink)发起人工作组以推动数据中心AI连接
- 星河云谷酒店亮相西安,开启“城市微度假”新空间
- 宝利化制药助力上海交通大学征战2024 iGEM
- 欧洲智慧能源展:双向充电可节省数十亿美元
- 中国酒业华夏奖揭晓,御粮喜获2023年度最具成长价值品牌
- 农发行湘潭市分行开展“爱助夕阳 陪伴有我”志愿服务活动
- 贵州苗知熠药业系列产品——《眸九州明眸亮眼草本精华》
- 中国美术名家走进罗浮山 李正天作品首展罗浮院子
- 广西服务企业百强榜揭晓,人民出行荣耀入围
- 第五届粤港澳大湾区文化创意设计大赛项目路演暨合作对接会成功举办
- Top 20 AI university graduates first Ph.D. cohort
- 创业新机遇,佰本本草开启健康消费新篇章
- The Apollo Group与Oaktree Capital合作促进下一阶段增长
- 凯丽亮相文荣奖颁发最佳男演员 献唱“一路凯歌”音乐会深情感人
- Wisson Robotics 通用软体机器人多功能应用在 CES 2025 上大放异彩
- Best-in-class just got better; Smith+Nephew announces 510(k) clearance of new CATALYSTEM™ Primary Hi
- WhatsApp脚本协议号注册工具,WS群发注册器让你注册更轻松
- 京能国际持续增长背后:融入“双碳”时代,迈向高质量发展
- GBCI report: Mainland China ranks among the most complex APAC jurisdictions in which to do business.
推荐
-
 透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
-
 王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
-
 私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
 中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
-
 海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
-
 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯

