New Data Strengthens Teva's Schizophrenia Portfolio
New Data Strengthens Teva's Schizophrenia Portfolio, Including Phase 3 SOLARIS Trial Survey Results Demonstrating Patient and Healthcare Professional Satisfaction with TEV-'749 (olanzapine) as a Once-Monthly Subcutaneous Long-Acting Injectable
- More than 92% of schizophrenia patients taking TEV-'749 in the SOLARIS survey were satisfied or very satisfied with the initiation regimen, dosing schedule and trial medication1
- New data from UZEDY® (risperidone) evaluated predictors of response in schizophrenia with efficacy observed across patient demographic and clinical characteristics in the Phase 3 RISE trial
- Teva continues its commitment to generating clinical insights that help advance and support optimal treatment for individuals living with schizophrenia
PARSIPPANY, N.J. and TEL AVIV, Israel, March 31, 2025 (GLOBE NEWSWIRE) -- Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd. (NYSE and TASE: TEVA), today announced the presentation of a patient and healthcare professional (HCP) attitudes and experiences survey study, complementing the successful Phase 3 Subcutaneous Olanzapine Extended-Release Injection Study (SOLARIS) evaluating TEV-'749. More than 92% of patients, 87% of nurses and 72% of physicians were either satisfied or very satisfied when asked about TEV-'749, overall, including initiation regimen, monthly dosing schedule and dosing options.1 The data were presented at the 2025 Congress of the Schizophrenia International Research Society (SIRS) taking place from March 29 to April 2, 2025, in Chicago, IL.
“Schizophrenia is an incredibly complex condition with profound impacts on mental health and daily life. That's why understanding the treatment perspectives of patients and healthcare providers is integral to Teva's research, and these survey results underscore the personal approach we've taken with the development of TEV-'749,” said Eric Hughes, MD, PhD, Executive Vice President, Global R&D and Chief Medical Officer at Teva. “This early clinical feedback on TEV-'749 is encouraging across some of the most important schizophrenia treatment factors – dosing schedule, initiation regimen, subcutaneous administration, post-injection monitoring requirements and general satisfaction.”
With nearly 30 years of clinical and real-world use, olanzapine is one of the most commonly prescribed second-generation oral antipsychotics for the treatment of schizophrenia globally, with a well-established efficacy and safety profile. Teva's Phase 3 SOLARIS trial program is evaluating the potential of TEV-'749 and its innovative drug delivery technology as a long-acting injectable (LAI) subcutaneous formulation of olanzapine that may also help address the risk of Post-Injection Delirium/Sedation Syndrome (PDSS).
“Olanzapine is foundational to the treatment of schizophrenia; however, I have seen first-hand the adherence challenges faced by patients and caregivers with daily oral options,” said Andrew J. Cutler, MD, Clinical Associate Professor of Psychiatry, SUNY Upstate Medical University and Chief Medical Officer, Neuroscience Education Institute. “The potential of TEV-'749 as a long-acting formulation of olanzapine that also may not carry a risk for PDSS and the accompanying risk evaluation & mitigation strategy (REMs) and post-injection observation period could be instrumental in helping to treat more people with schizophrenia.”
The prospective, cross-sectional, observational and online survey study recruited patient (n=70) and HCP participants (nurses: n=24; physicians: n=11) taking part in the SOLARIS trial who had two or more experiences with TEV-'749. The survey collected information on participants' demographics and clinical characteristics as well as their attitudes on and experiences with LAI treatment attributes, delivery of care, and treatment satisfaction.
Additional survey findings include:
- Injection-type preference: Patient preference for subcutaneous (SC) versus intramuscular (IM) injection (78.6% vs. 21.4%, respectively), with 67.3% of patients indicating the needle size as the main reason for their preference.1 For HCPs, SC versus IM preference was balanced for physicians (54.6% vs. 45.5%, respectively) and nurses (both 50.0%).1
- Post-injection monitoring requirements: Despite reporting low levels of social, emotional, financial or time impact, nearly all patients noted it would be helpful to have an LAI without a post-injection monitoring period (90.0%) or caregiver accompaniment requirement (92.9%).1 The majority of physicians (>90%) and nurses (>66%) responded that the post-injection monitoring period may present potential treatment barriers and clinical challenges that could impact patients' use of LAIs.1 Currently available olanzapine LAI has a three-hour post-injection monitoring requirement.2
- Initiation regimen: When starting an LAI, most participants preferred an initiation regimen requiring only one injection versus more complex regimens requiring concomitant oral medications or multiple injections (patients: 72.9% vs. 27.1%; physicians: 90.9% vs. 9.1%; nurses: 79.2% vs. 20.8%).1
- Dosing schedule: A majority of participants valued a monthly dosing schedule “a lot” (patients: 61.4%; physicians: 72.7%; nurses: 66.7%).1
- TEV-'749 satisfaction: Almost all participants had favorable responses (satisfied or very satisfied) regarding the initiation regimen, dosing schedule and trial medication overall (patients: >92%; physicians: >72%; nurses: >87%), and responded favorably regarding continuing TV-44749 (patients: 82.9%; physicians: 63.6%; nurses: 70.8%).1 Patients who had prior experience with LAIs were more likely to indicate they were “very satisfied” with trial medication overall (61.9% vs 53.1%). HCPs with more (≥10) TV-44749 experiences were more likely to rate it favorably (88.9% vs 76.5%) and be willing to continue treatment with TV-44749 (83.3% vs 52.9%).1
Below is the full set of schizophrenia data presented by Teva at SIRS 2025:
TEV-'749 (olanzapine):
- (De novo) Patient and healthcare professional attitudes and trial experiences with a subcutaneous long-acting injectable olanzapine (TV-44749) for the treatment of schizophrenia
UZEDY (risperidone):
- (De novo) Predictors of response and non-response to treatment for schizophrenia: machine learning analysis of patients treated with TV-46000 or placebo in the RISE study
Schizophrenia Treatment Landscape:
- (De novo) Healthcare professionals' attitudes toward use of long-acting injectable antipsychotics for schizophrenia treatment differ among settings of care: ADVANCE survey results
- (De novo) The evolving schizophrenia treatment landscape in the United States: A real-world claims analysis of treatment patterns and use of long-acting injectable antipsychotics
- (De novo) Real-world antipsychotic prescription patterns among patients with schizophrenia in Australia: Results from the ARIEL study
- (De novo) Country-specific factors influencing patients' willingness to use a long-acting injectable antipsychotic to treat schizophrenia: patient and caregiver ADVANCE survey results
- (De novo) Patient and caregiver engagement to support the development of clinical trials in adolescents living with schizophrenia
TEV-'749 is an investigational once-monthly subcutaneous LAI of the second-generation antipsychotic olanzapine and is not approved by any regulatory authority for any use, and its safety and efficacy are not established. The long-term safety of TEV-'749 and incidence of PDSS are being evaluated in the SOLARIS open-label study (Period 2).
TEV-'749 and UZEDY utilize SteadyTeq™, a copolymer technology proprietary to MedinCell that provides a controlled steady release of olanzapine and risperidone, respectively.
UZEDY was approved in the U.S. for the treatment of schizophrenia in adults in 2023.1
About Subcutaneous OLAnzapine Extended-Release Injection Study (SOLARIS)
SOLARIS is a multinational, multicenter, randomized, double-blind, parallel-group, placebo-controlled study to evaluate the efficacy, safety and tolerability of olanzapine extended-release injectable suspension for subcutaneous use as a treatment in patients (ages 18-65 years) with schizophrenia. For period one of the study (first 8 weeks), 675 patients were randomized to receive a subcutaneous injection of once-monthly TEV-'749 (low, medium or high dose) or placebo in a 1:1:1:1 ratio. For period two, which will last for up to 48 weeks, patients who completed period one are randomized and equally allocated to one of the three TEV-'749 treatment groups. The end-of-treatment and follow-up visits will be at 4 and 8 weeks after administration of the last treatment dose, respectively. The primary objective of the Phase 3 SOLARIS study was to evaluate the efficacy of TEV-'749 in adult patients with schizophrenia. A key secondary objective was to further evaluate the efficacy of TEV-'749 based on additional parameters in adult patients with schizophrenia. A secondary objective that is still ongoing through period two of the study is to evaluate the safety and tolerability of TEV-'749 in adult patients with schizophrenia.
About UZEDY
UZEDY (risperidone) extended-release injectable suspension for subcutaneous use is indicated for the treatment of schizophrenia in adults. In clinical trials, UZEDY significantly reduced the risk of schizophrenia relapse.1,3 UZEDY administers risperidone through copolymer technology under license from MedinCell that allows for rapid absorption and sustained release after subcutaneous injection. UZEDY is the only long-acting, subcutaneous formulation of risperidone available in both one- and two-month dosing intervals.3 For full prescribing information, visit https://www.uzedy.com/globalassets/uzedy/prescribing-information.pdf.
INDICATION AND USAGE
UZEDY (risperidone) extended-release injectable suspension for subcutaneous use is indicated for the treatment of schizophrenia in adults.
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. UZEDY is not approved for use in patients with dementia-related psychosis and has not been studied in this patient population.
See below for additional Important Safety Information.
IMPORTANT SAFETY INFORMATION CONTINUED
CONTRAINDICATIONS: UZEDY is contraindicated in patients with a known hypersensitivity to risperidone, its metabolite, paliperidone, or to any of its components. Hypersensitivity reactions, including anaphylactic reactions and angioedema, have been reported in patients treated with risperidone or paliperidone.
WARNINGS AND PRECAUTIONS
Cerebrovascular Adverse Reactions: In trials of elderly patients with dementia-related psychosis, there was a significantly higher incidence of cerebrovascular adverse events (e.g., stroke, transient ischemic attack), including fatalities, in patients treated with oral risperidone compared to placebo. UZEDY is not approved for use in patients with dementia-related psychosis.
Neuroleptic Malignant Syndrome (NMS): NMS, a potentially fatal symptom complex, has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status including delirium, and autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure. If NMS is suspected, immediately discontinue UZEDY and provide symptomatic treatment and monitoring.
Tardive Dyskinesia (TD): TD, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements, may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to predict which patients will develop the syndrome. Whether antipsychotic drug products differ in their potential to cause TD is unknown.
The risk of developing TD and the likelihood that it will become irreversible are believed to increase with the duration of treatment and the cumulative dose. The syndrome can develop, after relatively brief treatment periods, even at low doses. It may also occur after discontinuation. TD may remit, partially or completely, if antipsychotic treatment is discontinued. Antipsychotic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome, possibly masking the underlying process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
If signs and symptoms of TD appear in a patient treated with UZEDY, drug discontinuation should be considered. However, some patients may require treatment with UZEDY despite the presence of the syndrome. In patients who do require chronic treatment, use the lowest dose and the shortest duration of treatment producing a satisfactory clinical response. Periodically reassess the need for continued treatment.
Metabolic Changes: Atypical antipsychotic drugs have been associated with metabolic changes that may increase cardiovascular/cerebrovascular risk. These metabolic changes include hyperglycemia, dyslipidemia, and body weight gain. While all of the drugs in the class have been shown to produce some metabolic changes, each drug has its own specific risk profile.
Hyperglycemia and diabetes mellitus (DM): in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, have been reported in patients treated with atypical antipsychotics, including risperidone. Patients with an established diagnosis of DM who are started on atypical antipsychotics, including UZEDY, should be monitored regularly for worsening of glucose control. Patients with risk factors for DM (e.g., obesity, family history of diabetes) who are starting treatment with atypical antipsychotics, including UZEDY, should undergo fasting blood glucose (FBG) testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics, including UZEDY, should be monitored for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients who develop symptoms of hyperglycemia during treatment with atypical antipsychotics, including UZEDY, should undergo FBG testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic, including risperidone, was discontinued; however, some patients required continuation of antidiabetic treatment despite discontinuation of risperidone.
Dyslipidemia has been observed in patients treated with atypical antipsychotics.
Weight gain has been observed with atypical antipsychotic use. Monitoring weight is recommended.
Hyperprolactinemia: As with other drugs that antagonize dopamine D2 receptors, risperidone elevates prolactin levels and the elevation persists during chronic administration. Risperidone is associated with higher levels of prolactin elevation than other antipsychotic agents.
Orthostatic Hypotension and Syncope: UZEDY may induce orthostatic hypotension associated with dizziness, tachycardia, and in some patients, syncope. UZEDY should be used with particular caution in patients with known cardiovascular disease, cerebrovascular disease, and conditions which would predispose patients to hypotension and in the elderly and patients with renal or hepatic impairment. Monitoring of orthostatic vital signs should be considered in all such patients, and a dose reduction should be considered if hypotension occurs. Clinically significant hypotension has been observed with concomitant use of oral risperidone and antihypertensive medication.
Falls: Antipsychotics, including UZEDY, may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls and, consequently, fractures or other fall-related injuries. Somnolence, postural hypotension, motor and sensory instability have been reported with the use of risperidone. For patients, particularly the elderly, with diseases, conditions, or medications that could exacerbate these effects, assess the risk of falls when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
Leukopenia, Neutropenia, and Agranulocytosis have been reported with antipsychotic agents, including risperidone. In patients with a pre-existing history of a clinically significant low white blood cell count (WBC) or absolute neutrophil count (ANC) or a history of drug-induced leukopenia or neutropenia, perform a complete blood count (CBC) frequently during the first few months of therapy. In such patients, consider discontinuation of UZEDY at the first sign of a clinically significant decline in WBC in the absence of other causative factors. Monitor patients with clinically significant neutropenia for fever or other symptoms or signs of infection and treat promptly if such symptoms or signs occur. Discontinue UZEDY in patients with ANC < 1000/mm3) and follow their WBC until recovery.
Potential for Cognitive and Motor Impairment: UZEDY, like other antipsychotics, may cause somnolence and has the potential to impair judgement, thinking, and motor skills. Somnolence was a commonly reported adverse reaction associated with oral risperidone treatment. Caution patients about operating hazardous machinery, including motor vehicles, until they are reasonably certain that treatment with UZEDY does not affect them adversely.
Seizures: During premarketing studies of oral risperidone in adult patients with schizophrenia, seizures occurred in 0.3% of patients (9 out of 2,607 patients), two in association with hyponatremia. Use UZEDY cautiously in patients with a history of seizures or other conditions that potentially lower the seizure threshold.
Dysphagia: Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. Antipsychotic drugs, including UZEDY, should be used cautiously in patients at risk for aspiration.
Priapism has been reported during postmarketing surveillance for other risperidone products. A case of priapism was reported in premarket studies of UZEDY. Severe priapism may require surgical intervention.
Body temperature regulation. Disruption of the body's ability to reduce core body temperature has been attributed to antipsychotic agents. Both hyperthermia and hypothermia have been reported in association with oral risperidone use. Strenuous exercise, exposure to extreme heat, dehydration, and anticholinergic medications may contribute to an elevation in core body temperature; use UZEDY with caution in patients who experience these conditions.
ADVERSE REACTIONS
The most common adverse reactions with risperidone (≥5% and greater than placebo) were parkinsonism, akathisia, dystonia, tremor, sedation, dizziness, anxiety, blurred vision, nausea, vomiting, upper abdominal pain, stomach discomfort, dyspepsia, diarrhea, salivary hypersecretion, constipation, dry mouth, increased appetite, increased weight, fatigue, rash, nasal congestion, upper respiratory tract infection, nasopharyngitis, and pharyngolaryngeal pain.
The most common injection site reactions with UZEDY (≥5% and greater than placebo) were pruritus and nodule.
DRUG INTERACTIONS
- Carbamazepine and other strong CYP3A4 inducers decrease plasma concentrations of risperidone.
- Fluoxetine, paroxetine, and other strong CYP2D6 inhibitors increase risperidone plasma concentration.
- Due to additive pharmacologic effects, the concomitant use of centrally-acting drugs, including alcohol, may increase nervous system disorders.
- UZEDY may enhance the hypotensive effects of other therapeutic agents with this potential.
- UZEDY may antagonize the pharmacologic effects of dopamine agonists.
- Concomitant use with methylphenidate, when there is change in dosage of either medication, may increase the risk of extrapyramidal symptoms (EPS).
USE IN SPECIFIC POPULATIONS
Pregnancy: May cause EPS and/or withdrawal symptoms in neonates with third trimester exposure. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics, including UZEDY, during pregnancy. Healthcare providers are encouraged to register patients by contacting the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or online at http://womensmentalhealth.org/clinicaland-research-programs/pregnancyregistry/.
Lactation: Infants exposed to risperidone through breastmilk should be monitored for excess sedation, failure to thrive, jitteriness, and EPS.
Fertility: UZEDY may cause a reversible reduction in fertility in females.
Pediatric Use: Safety and effectiveness of UZEDY have not been established in pediatric patients.
Renal or Hepatic Impairment: Carefully titrate on oral risperidone up to at least 2 mg daily before initiating treatment with UZEDY.
Patients with Parkinson's disease or dementia with Lewy bodies can experience increased sensitivity to UZEDY. Manifestations and features are consistent with NMS.
Please see the full Prescribing Information for UZEDY, including Boxed WARNING.
About Teva
Teva Pharmaceutical Industries Ltd. (NYSE and TASE: TEVA) is a different kind of global biopharmaceutical leader, one that operates across the full spectrum of innovation to reliably deliver medicines to patients worldwide. For over 120 years, Teva's commitment to bettering health has never wavered. Today, the company's global network of capabilities enables its 37,000 employees across 57 markets to advance health by developing medicines for the future while championing the production of generics and biologics. We are dedicated to addressing patients' needs, now and in the future. Moving forward together with science that treats, inspired by the people we serve. To learn more about how Teva is all in for better health, visit www.tevapharm.com.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, which are based on management's current beliefs and expectations and are subject to substantial risks and uncertainties, both known and unknown, that could cause our future results, performance or achievements to differ significantly from that expressed or implied by such forward-looking statements. You can identify these forward-looking statements by the use of words such as “should,” “expect,” “anticipate,” “estimate,” “target,” “may,” “project,” “guidance,” “intend,” “plan,” “believe” and other words and terms of similar meaning and expression in connection with any discussion of future operating or financial performance. Important factors that could cause or contribute to such differences include risks relating to: our ability to successfully develop TEV-‘749 (olanzapine LAI) in adult patients diagnosed with schizophrenia; our ability to successfully develop and commercialize UZEDY (risperidone) extended-release injectable suspension for the treatment of schizophrenia; our ability to successfully compete in the marketplace, including our ability to develop and commercialize additional pharmaceutical products; our ability to successfully execute our Pivot to Growth strategy, including to expand our innovative and biosimilar medicines pipeline and profitably commercialize the innovative medicines and biosimilar portfolio, whether organically or through business development; and other factors discussed in our Annual Report on Form 10-K for the year ended December 31, 2024, including in the section captioned “Risk Factors” and “Forward Looking Statements.” Forward-looking statements speak only as of the date on which they are made, and we assume no obligation to update or revise any forward-looking statements or other information contained herein, whether as a result of new information, future events or otherwise. You are cautioned not to put undue reliance on these forward-looking statements.
- Data on file. Parsippany, NJ: Teva Neuroscience, Inc.
- Detke HC, McDonnell DP, Brunner E, Zhao F, Sorsaburu S, Stefaniak VJ, Corya SA. Post-injection delirium/sedation syndrome in patients with schizophrenia treated with olanzapine long-acting injection, I: analysis of cases. BMC Psychiatry. 2010 Jun 10;10:43.
- UZEDY (risperidone) extended-release injectable suspension, for subcutaneous injection Current Prescribing Information. Parsippany, NJ. Teva Neuroscience, Inc.
Teva Media Inquiries:
TevaCommunicationsNorthAmerica@tevapharm.com
Teva Investor Relations Inquires
TevaIR@Tevapharm.com
- 《大江大河之岁月如歌》完美收官,时代见证王凯与宋运辉共成长
- 林达烈士牺牲77周年座谈会暨数字人发布仪式在福寿园海港陵园举行
- 镭神智能3D SLAM无人叉车,激光雷达如何铸就立体安全防护
- 临商银行北京路支行开展“讲最满意的一件事和最不满意的事”主题党日活动
- 文十贰影视悬疑奇幻短剧《半岛大厦怪谈》在渝开机
- 广州服装培训学院服装陈列与展示设计技能课程_广州服装设计学院
- 矿用阻燃网线MHSYV-5-420.5:欧孚光电厂家守护神5
- 五新装备小断面利器星耀上海宝马展 展现中国智造非凡魅力
- 支付宝电商平台助力家庭服务升级——栀美家政服务部,让生活更美好
- 索斯科推出新一代拴式螺钉
- 丁春亚|龙泉鼎丰温德姆酒店:一剑一瓷,一城一梦
- Instagram营销软件,ins群发工具,ig批量私信神器/ins一手协议号
- 临商银行成都路支行举办实物贵金属业务营销培训
- “我爱我的祖国•童筑中国梦”少儿才艺电视展演活动启动
- 2023河北省优秀基层文艺院团文化惠民演出活动圆满结束
- 你的随身网络战甲来了!飞猫与王者荣耀合作款U8随身WiFi全网开售
- 千寻位置携手中海达推出联名款RTK 开创行业合作新模式
- 世界和平与可持续发展基金会中国办事处筹备会在京召开
- 马丽果然天生就是吃演员这碗饭的,《抓娃娃》中她把春兰演得太对味儿了
- 平安养老险安徽分公司提醒您:警惕以“定制蛋糕”为幌子,利用商户账户洗钱
- Ascom Annual General Meeting approves increased dividend payout of CHF 0.30
- Beyonttra™ (acoramidis), the First Near-complete TTR Stabilizer (≥90%), Approved in Japan to Treat A
- 乘风出海!CGT绿城体育成为英超豪门热刺俱乐部人造草坪官方供应商
- “精彩2024· LEGEND演唱会”金华站官宣,华语乐坛顶级唱将齐聚金华
- WS抓住商机脉搏,WhatsApp营销工具是你紧跟趋势的最佳伙伴
- 南京肤康医院骗了多少人?事实粉碎 “欺骗” 谣言
- WS数字之花盛放!WhatsApp营销工具,为你的销售数字灌溉奇迹之水
- 因地制宜下真功夫 中国人寿财险助力民营经济、中小微企业大有可为
- “护航新征程 存款伴您行”临商银行成都路支行多渠道开展存款保险宣传
- 个人股权转让涉税热点及自行申报操作指南
推荐
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
-
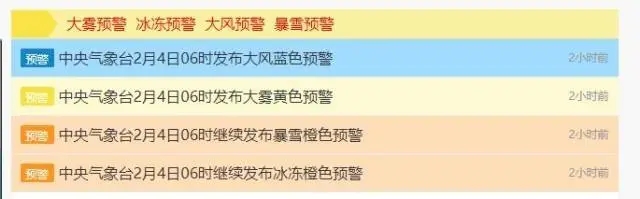 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
-
 王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
-
 透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
-
 新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
-
 私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
-
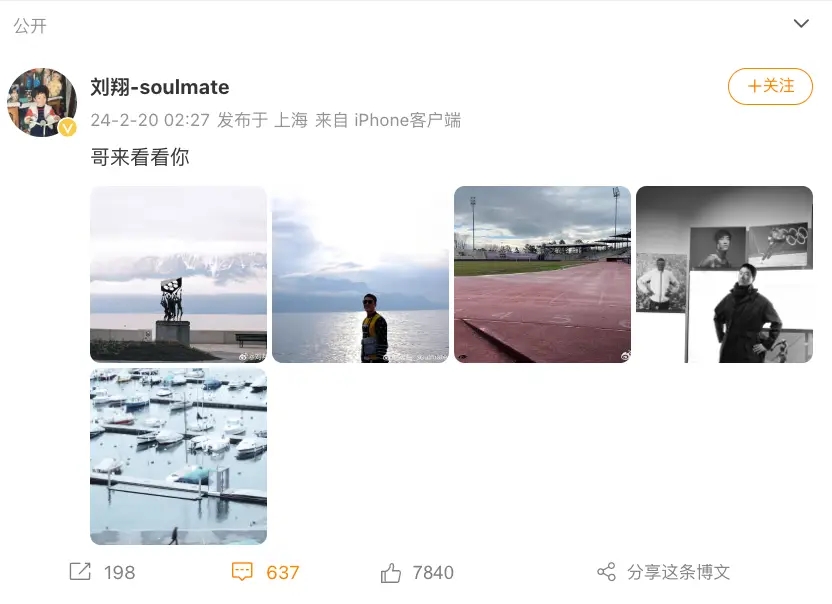 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯

